Chemical Depilatories
Chemical depilatories are one of the most common methods used to remove superfluous and other unwanted hair. They function by disintegrating hair into an amorphous mass which is then scraped, wiped or rinsed off the skin. Their primary effect is on the keratin protein that makes up the hair shaft. Unfortunately, as the surface of the skin is also largely made of keratin, depilatories can have an irritating effect on it as well.
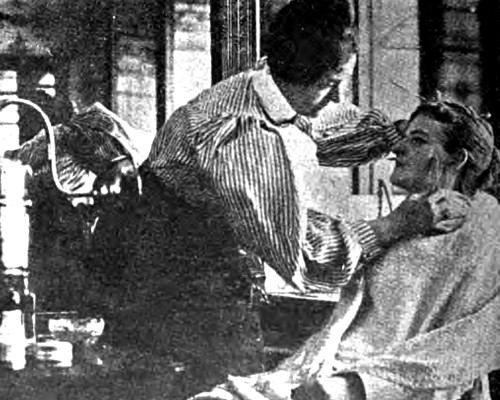
Above: 1905 Applying a depilatory to remove ‘sideburns’.
See also: Hair Removers
Keratin owes a good deal of its structure to the disulphide bonds that occur between the cystine amino acids that make up its structure – hair keratin contains about 14% cystine, skin keratin less than 3%. These disulphide bonds hold the polypeptide chains of the keratin protein together giving it elasticity and strength. The bonds are very strong but can be broken by heat or chemicals – typically reducing agents in an alkaline pH.
Depilatories share some commonality with two other cosmetic products: permanent waving solutions and cuticle removers. The use of reducing agents to break the disulphide bonds also underlies the basic chemistry behind the cold permanent wave, and some of the chemicals found in later depilatories were also used to ‘perm’ hair. Their high (alkaline) pH means that depilatories also have a functional similarity with cuticle removers as both products use an alkali to a soften keratin and make its removal easier.
Rhusma
The earliest known chemical depilatory that was widely used in Europe originated in the Middle East. Known as rhusma or rusma, it was a paste made of slaked lime and orpiment (natural arsenic trisulphide) mixed together with water.
Celnart was a courageous advocate of cosmetics, or else she was wise enough to put the worst first, for one of her earliest recipes is this depilatory, which is not at all quoted by way of recommendation. It is the Oriental Rusma, a depilatory used in harems:
Two ounces of quicklime, half an ounce of orpiment and red arsenic; boil in one pint of alkaline lye, and try with a feather to see when it is strong enough. Touch the parts to be rid of hair, and wash with cold water.(Ugly girl papers, 1874, p. 139)
The word depilatory is derived from de pilus of the hair. As the ladies of this country consider the growth of hair upon the upper lip, upon the arms, and on the back of the neck to be detrimental to beauty, those who are troubled with such physical indications of good health and vital stamina have long had recourse to rusma or depilatory for removing it. This or analogous preparations were introduced into this country from the East, rusma having been in use in the harems of Asia for many ages.
Best lime slaked 3 lbs. Orpiment, in powder ½ lbs. Mix the material by means of a drum sieve; preserve the same for sale in well corked or stoppered bottles.
Directions to be sold with the above:—
Mix the depilatory powder with enough water to render it of a creamy consistence; lay it upon the hair for about five minutes, or until its caustic action upon the skin renders it necessary to be removed; a similar process to shaving is then to be gone through.(Piesse, 1862, p. 313)
Although rhusma is still employed as a depilatory in some parts of the world today, by the middle of the nineteenth century its use in Europe began to be superseded by less dangerous sulphides. This is not to suggest that rhusma disappeared completely from the Western World by 1900. Ruth D. Maurer [1871-1945], who founded the Marinello company in the United States under the name Emily Lloyd, was still advocating the use of rhusma in 1907, although she did not name it as such.
[T]he depilatory given here may be tried, and though said to be extremely effective in some cases, it is certainly a trifle severe in others and can never be applied with any degree of certainty as far as the after-effect on the skin is concerned.
DEPILATORY Orpiment 1 ℥ Quicklime 10 ℥ Starch 14 ℥ The ingredients must be ground into a fine powder and kept in a tightly corked bottle. When used, a small portion of the powder should be mixed with water into a paste and spread over the hairy portions. As soon as the paste dries, wash it off, and the hair will come with it. If the skin is extremely sensitive and commences to smart, the paste will need to be removed immediately or the cuticle will be injured. In any event, as soon as it has been washed off, a mild cream should be applied.
(Lloyd, 1907, pp. 176-177)
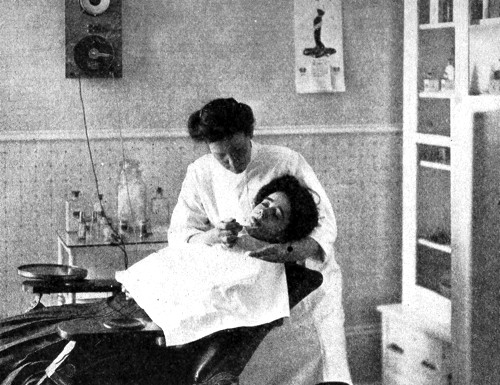
Above: 1907 Applying a depilatory to the chin in a Marinello salon.
See also: Marinello
I also note that Kathleen Mary Quinlan was selling a depilatory she called rusma well into the 1920s. Unfortunately, I do not know its formulation so it may not have been made using slaked lime and orpiment.
See also: Kathleen Mary Quinlan
Sulphides
The use of rhusma as a depilatory relies on the fact that orpiment reacts with lime to form a soluble sulphide. However, as both arsenic and antimony sulphide are highly poisonous, they cannot be used with any safety. Other sulphides were also ruled out, either because they are not soluble in water or were too feeble in action. This left the sulphides of sodium, barium, strontium and calcium remaining – listed in decreasing order of solubility and causticity (Koeune, 1937, p. 15) – and these formed the basis of most commercial depilatories produced to the 1940s.
The first recorded use of one of these sulphides as a depilatory comes from Septimus Piesse, who notes in the 1857 edition of his work, that a Dr. Redwood recommended using barium sulphide to remove hair.
Dr. Redwood says that the best and safest depilatory consists of a strong solution of sulphuret of barium made into a paste with thick starch: it must be applied immediately it is made as it rapidly spoils.
(Piesse, 1862, p. 314)
Some sulphide depilatories were covered by patents that limited their commercial use for a time. Jacques Perl, for example, took out a patent for a depilatory made from strontium sulphate in France (FR201,778: 1889), Great Britain (GB8,029: 1889), Germany (DE54,127: 1889), and the United States (US450,032: 1891).
Numerous experiments have shown that the soluble strontic salts do not in any wise manifest toxical or irritating qualities. The depilatories prepared from the aforesaid sulphurated derivatives of strontium are therefore perfectly harmless to the human body and agreeable in use.
(U.S. Patent 450,032, 1891)
Although a patent would stop the commercial production of a depilatory covered by the patent, it would not stop someone from making up their own mixture and it is probable that many beauty salons did just that.
As a depilatory application for the temporary removal of superfluous hair, barium and strontium sulphide heads the list. I prefer strontium, as it is less toxic, and differs from other depilatory agents in not evolving hydrogen sulphide. My favorite formula is as follows:
Strontium Sulphide 30 gr. Zinc Oxide 15 gr. Starch 15 gr. Triturate thoroughly and mix sufficient water to make a paste. This is applied over the surface containing the superfluous hair and allowed to remain five or ten minutes, when it can be removed by scraping the surface with some blunt knife similar to a paper knife, or it may be rubbed off with absorbent cotton; the face should be washed, cleaned and some bland oil applied.
(Covey, 1905, p. 246)
Although Perl’s patent for the use of strontium sulphate lapsed after 17 years, and strontium sulphide was recommended by cosmetic chemists, many commercial depilatories sold in the United States continued to use other forms, possibly because strontium sulphide was more expensive.
Delatone.—This powder is typical of that class of preparations called depilatories that are used for removing hair. They depend for any action they may have upon an alkaline sulphide such as sodium, calcium or barium and the principal objection to them is the extravagant price charged. This particular one is composed of barium sulphide 7%, barium sulphate 7% and starch 85%. The ingredients of a dollar package are worth about one cent.
Delol.—A depilatory manufactured by the P. W. Scharff Company which contains:
Barium Sulphate 14% Barium Sulphide 14% Sulphur 4% Calcium Carbonate 3% Zinc Oxide 17% Starch 48% (For remarks see Delatone.)
De Miracle.—A depilatory solution of sodium sulphide in water. The contents of a dollar package are worth but one or two cents. (See Delatone.) …
Depilatory.—This powder is manufactured by H. Hatten of Chicago and contains:
Sulphur 7% Barium Sulphate 25% Barium Sulphide 21% Calcium Carbonate 10% Starch 37% (See Delatone.)
Depilatory.—Harriet Hubbard Ayers of New York City also manufactures a depilatory that has the following composition:
Barium Sulphide 32% Starch 68% (See Delatone.) …
El Rado.
Sodium Sulphide 5% Glycerine 10% Water 85% On-Riah.—Another depilatory containing about three cents worth of ingredients that is sold for fifty cents. The composition is as follows:
Barium Sulphide 43% Talc 17% Starch 40% (Indiana State Board of Health, 1917, pp. 71-77)
The situation in America was not necessarily duplicated elsewhere. For example, in the United Kingdom barium sulphide was put on the U.K. Poisons Schedule which meant that products containing it could only be sold through pharmacies. Beauty culturists in the U.K. could still get hold of barium sulphide and use it to mix up a depilatory for use in their salon but it limited the sales of any commercial depilatory that used it, so manufacturers there looked at alternatives.
A simple paste contains the following ingredients—
Sulphuret of barium (in solution)
Powdered starch
Enough powdered starch to form a paste with the sulphuret of barium is the right proportion, and this must be applied to the skin, left on for about three minutes, and then scraped off, when the hairs are removed in the hardened paste.(Verni, 1946, p. 439)
Manufacturing considerations
Sulphide depilatories form hydroxides and hydrosulphides when added to water.
Sodium Sulphide + Water = Sodium Hydroxide = Sodium Hydrosulphide
The hydrosulphides react with the hair to break down the disulphide bonds while the hydroxides (which are alkaline) help cause the hair to absorb water, so that it swells and softens, making it easier for the hydrosulphides to penetrate the hair.
Despite numerous advertising claims to the contrary, skin irritation was a major problem for sulphide depilatories and the primary reason for complaints which occasionally led to claims for compensation after the skin became ulcerated. Irritation was also one of the reasons why many women went back to shaving or waxing.
One way manufacturers could reduce irritation was in the selection of the sulphide they used. Very soluble hydroxides are more irritating than less soluble forms so, as sodium hydroxide is more soluble than either barium or calcium hydroxide it is potentially more irritating to the skin. Therefore, selecting to use either barium or calcium sulphide in preference to sodium sulphide could help reduce the irritating effect of the depilatory.
Glycerin, or some other glycol, was also used by some manufacturers to reduce irritation. Glycols had the additional advantage of improving the stability of a depilatory, giving it a longer shelf life (deNavarre, 1941, p. 537).
The concentration of the sulphide was also an important consideration for skin irritability. From 4-5 minutes was considered a safe time for a sulphide depilatory to act (deNavarre, 1941, p. 543). This was not a simple matter. A faster time could mean that the depilatory was too strong and could irritate the skin but a slower time, as well as being impractical, also gave the depilatory more time to irritate the skin.
The coarseness of the hair also affected the time needed for the product to work. This differed across the various parts of the body and from person to person so careful manufacturers would need to determine the optimal time required for their depilatory to work on fine, medium and coarse hair and place this information on their product packaging. Unfortunately, coarser hair could be found on sensitive areas of the body so leaving the depilatory on for longer could become a major problem in regions such as under the arms (axilla).
When making a sulphide depilatory manufacturers also had to consider its colour. Most sulphides are an unattractive yellow or bluish-green caused by the formation of coloured sulphides, particularly iron sulphide. Fortunately this problem could be easily covered by adding white powders like zinc oxide, titanium dioxide or zinc sulphide to the mix.
Sulphide depilatories also release varying amounts of hydrogen sulphide (rotten egg gas) when in solution.
Sodium hydrosulphide + Water = Sodium Hydroxide + Hydrogen Sulphide gas
To reduce the bad smell manufacturers could add absorbents like gums, carbon and clays to draw in and hold the released gas, and/or partially cover it with perfume. Adding a perfume was not without its difficulites, not only because of the strength of the rotten-egg gas but also because sulphides are highly reactive. They had an adverse effect on many aromatic compounds making them unsuitable for use.
Powders, liquids and creams
Prior to the 1920s, sulphide depilatories were sold as powders, liquids or lotions with creams (technically pastes) first appearing in the 1920s.

Above: 1935 Different forms of chemical depilatory: 4711 (powder), DeMiracle (liquid) and Neet (cream).
The powders were easy to manufacturer and transport, making them ideal for sale by postal order. They were usually packaged in bottles sealed with a cork to keep the powder in and the moisture out. They required the user to mix a measured quantity of powder with water to form a paste so were not particularly convenient – a wooden spatula was often included with the packaging as the sulphides reacted unfavourably with metal.
Liquid and lotion depilatories could be used directly from the bottle but, being liquids, they were more difficult to constrain to defined areas of the skin and were not as chemically stable as powders. Exposure to the air caused them to deteriorate which meant that their shelf life was often an issue.
Cream (paste) depilatories were introduced in the 1920s. From 1921, Joseph Donner took out a series of patents for a cream depilatory in the United States (US1,379,855: 1921); France (FR52,622: 1921); and Great Britain (GB163,022: 1922). Donner’s patents were for a sulphide depilatory in an air-restraining cream which included a gum such as agar, tragacanth, acacia or quince seed. This not only made applying the depilatory very easy – without the risk of it running or spreading – but as the gums absorbed the hydrogen sulphide the levels of objectionable odours were lower. Donner had therefore produced a stable depilatory cream with none of the usual defects that normally go with either the powder or liquid/lotion forms. The idea was copied and although Donner defended his patents, the advantages inherent in creams (pastes) saw them become the preferred form for depilatories thereafter.
As many gums were affected by the alkaline medium they were later largely replaced by other absorbents including carbon particles and clays.
Cream depilatories are misnamed. They should be called paste depilatories, since they are solid suspensions in a water solution, the proportion of solids to liquid being regulated to give a semi-solid mass. The active ingredient is one of the alkaline earth sulfides—barium sulfide, strontium sulfide or calcium sulfide. The percentage of sulfide is fairly low; so a filler is needed. In the case of calcium sulfide this is usually slaked lime, although as with others it may be an inert filler such as clay, starch or precipitated chalk. These materials serve another aim: with surface absorption of the hydrogen sulfide the odor becomes less. (Stronger gas absorbents, such a specially treated carbons and clays, have been added for this purpose.) These inert fillers also act as perfume carriers, partly to mask the sulfide odor with a heavier but more pleasing odor.
(McDonough, 1937, p. 196)

Above: 1940 Zip Hair Removing Lotion. Although a cream became the preferred depilatory form other types persisted on the market. This one has an applicator built into the lid.
Stannites
A second group of chemicals used as depilatories were the stannites (stannous (tin) hydroxides). Used mainly in the 1930s and 1940s, their main advantage was that, unlike the sulphides, they had no appreciable odour. However, they were not without their own idiosyncrasies, the main issue being they were unstable unless kept in a strongly alkaline solution. This made any depilatory that used them potentially very irritating to the skin. Cosmetic chemists attempted to solve this difficulty by adding chemical stabilisers, a number of which were patented. Some stabilisers enabled the alkalinity of the depilatory to be lowered thereby reducing any potential irritability.
The usefulness of stannite depilatories declined rapidly after the introduction of thioglycolates in the 1940s.
Thallium acetate
In 1912, Dr. Raymond Sabouraud [1864-1938], a French authority on scalp disease, suggested that thallium acetate could be used to remove superfluous hair. The report generated a good deal of interest, particularly as, unlike other depilatories, it was said to prevent regrowth.
Preparation for the Removal of Hair
Strictly speaking, the application of chemical substances is useless, inasmuch as the hair always grows again. Electrolysis aims at the destruction of the roots of the hair, and is quite effective when properly done; it is comparatively painless, and does not blemish the skin, but should be carried out by an expert. The following particulars regarding a thallium acetate depilatory may be of interest:–
Thallium acetate 0.30 gns. Zinc Oxide 2.50 gns. Soft paraffin 20.00 gns. Lanoline 5.00 gns. Rose water 5.00 gns. A small piece of this ointment, no larger than the bulk of 1 or 2 grains of wheat, is applied to the lips (or any other affected part) every night. This suffices to bring about the destruction of the hair or down. In those cases where the hair is coloured and about half an inch long, its disappearance is rapid. It is naturally replaced, however, by fresh growth, but the regular use of the ointment is said to ensure its remaining short and almost invisible.
(Hairdressing, 1913, p. 1070)
The depilating effect of thallium acetate had been known since 1897 and Sabouraud had been experimenting with it from about 1910 as a treatment for ringworm.
Unlike other chemical depilatories, thallium acetate did not produce an immediate hair loss and results only became evident after six or seven days. The reason for this was that thallium is not a keratolytic but rather is a poison – only slightly less toxic than arsenic – one of the symptoms of thallium poisoning being hair loss.
The most famous thallium acetate depilatory was Koremlu Cream first marketed in 1930 by the Koremlu company founded by Kora M. Lubin – the company name being an abbreviation of her name.

Above: 1915 Korozone. This advertisement indicates that Kora M. Lubin had been in the hair removal business well before the Koremlu company was founded. The product name suggests that in this case she was providing some sort of ozone treatment.
The initial Koremlu product contained 3.25% thallium acetate but this was later increased to between 5% and 7% to make it more effective. In 1931, the American Medical Association (AMA) began receiving reports of thallium poisoning after using Kormulu. The product was then banned in some states. The company filed for bankruptcy in July, 1932, removing the possibility of users receiving compensation, but also eliminating further production. Damage suits filed against it totaled US$2,500,000 but the company had only US$5 in assets (Kallett & Schlink, 1933, p. 87).
Also see the booklet: The Koremlu Cream Method (1931)
Despite the obvious dangers of using the compound, not all cosmetic chemists were initially convinced that its use should be banned. In 1935 for example, in reply to some criticism from Frank Atkins, Harold Stillman wrote:
On the question of thallium acetate, I have yet to be convinced that this is not one of the best methods of depilatory treatment. Since it is not a keratolytic, it has no destructive action on the skin, and it is not merely a palliative, but a genuine depilatory. … Of course thallium acetate is a poison, and for this reason I laid special stress on the fact that it must be used intelligently. … any number of beauty specialists in this country, and even more on the continent and in America, apply the thallium acetate treatment successfully. Perhaps it is all a matter of education.
(Atkins, 1935, pp. 13, 10)
Thioglycolates
A major advance in the formulation of depilatories came with the introduction of thioglycolates, also known as mercaptans. These chemicals would have a impact on Beauty Culture that extended far beyond depilatories as they were also used to develop the first cold permanent wave in 1938.
The action of thioglycolates was discovered In the early 1930s when David Rockwell Goddard [1908-1985], at the Rockefeller Institute in New York, demonstrated that the structure of protein could be degraded by breaking its disulphide bonds with thioglycolic acid. Goddard did not patent this discovery leaving an opening for others to do so.
The first use of thioglycolic acid as a depilatory was industrial. In 1934, the Röhm & Haas Company was granted a patent for the use of thioglycolic acid to dehair animal hides. Soon after this other patents were issued for human depilatories using thioglycolates, with those by Karel Bohemen in Great Britain (GB484467: 1938) and William Fletcher in France (FR824,804: 1938) being the earliest.
The most wide reaching patents for thioglycolic depilatories were granted to Ralph Evans and Everett McDonough (FR844,529: 1939; GB521,240: 1940; US2,352,524: 1944; GB593,438: 1945). These patents were vigorously defended in the courts. In 1947, Sales Affiliates, Inc. – who owned the Evans and McDonough patents – took out successful injunctions against Sleek (Elizabeth Arden) and Nair (Carter Products) on the grounds that these depilatories infringed their patents. Sales Affiliates also licensed other companies – such as Imra (Schering Corporation) and X-Bazin (Hall & Ruck) to use the Evans and McDonough patent (AP&EOR, 1947).
Thioglycolates had a number of advantages over the older sulphide depilatories. They were more stable, less likely to react with perfumes, did not discolour when exposed to metals – so formed a pure white cream – and best of all were odour free.
Manufacturing considerations
Early thioglycolate depilatories were pastes, containing clays and methylcellulose, or stearyl alcohol and alkyl sulphate (deNavarre, 1975, p. 1252).
Strontium hydrate 50 gms. Calcium Oxide 12 gms. Colloidal clay 102 gms. Methyl cellulose 11 gms. Perfume 0.8 cc. Water 300 cc. By the addition of 12 ccs. of thioglycerol to the ingredients of the above formula, a depilatory may be prepared having the important improved characteristics and novel advantages … The thioglycerol content may be varied within the range of 9-18 cc.
(British patent No. 593,438, 1945)
As thioglycolates rapidly oxidise when exposed to the air, the finished preparation needs to be stored in an airtight container. This means that commercial preprations are mostly sold as creams or pastes in collapsible tubes. However, as potassium and calcium thioglycolate-based depilatories were relatively slow acting, men who used a depilatory in place of shaving generally mixed it up from a powder.
The facial hair of the black male is often curly and wiry. Shaving leaves the exposed ends of the hairs with sharp points, and as the hairs regrow these sharp points can actually turn back onto and penetrate the skin, causing a clinical condition called pseudofolliculitis barbae. For this reason some black men prefer to use a depilatory that not only gives a closer “shave” but also leaves the hair tip soft and blunt so that it does not puncture and reenter the skin.
Conventional thioglycolate-based depilatories take 15-20 minutes to remove beard hair, which is regarded as far too long. Effectiveness outweighs cosmetic elegance, and the tendency is to use a powder depilatory that must be mixed with water before use but which provides adequate hair removal in 3-7 minutes. In the United States the active ingredient commonly used in powder depilatories is calcium thioglycolate, which exhibits low odor and is easily perfumed.(Reiger, 2000, p. 721)
Given their inherent advantages, it is not difficult to see why thioglycolates – such as potassium thioglycolate and calcium thioglycolate – have become the basis of most modern depilatories. Made mostly as a creamy emulsion, they normally include an alkali, such as sodium hydroxide or calcium hydroxide, to help soften the hair, and a wetting agent, such as Ceteareth-20, to make it easier for the thioglycolate to penetrate and act. Other ingredients are added to soothe the skin, to function as an antiseptic, or to add colour or fragrance.
Veet: Deionized Water, Cetearyl Alcohol, Urea, Potassium Thioglycolate, Ceteareth-20, Mineral Oil, Calcium Hydroxide, Sodium Magnesium Silicate, Magnesium Trisilicate, Shea Butter, Fragrance, Titanium Dioxide, Propylene Glycol, Sodium Gluconate, Acrylates Copolymer.
Nair: Water, Mineral Oil, Calcium Hydroxide, Urea, Potassium Thioglycolate, Cetearyl Alcohol, Ceteareth-20, Sodium Hydroxide, Camellia Oleifera Extract, Sunflower Seed Oil, Fragrance, Chromium Hydroxide Green.
First Posted: 31st March 2014
Last Update: 29th July 2022
Sources
The American perfumer & essential oil review. (1906-1955). New York: Robbins Perfumer Co. [etc.].
Arend, A. G. (1934). Depilatory manufacture. The Perfumery and Essential Oil Record, September, 266-268.
Atkins, F. (1935). Progress in depilatories—A criticism. The Manufacturing Chemist, January, 11-13, 10.
Barry, R. H. (1957). Depilatories. In E. Sagarin (Ed.). Cosmetics: Science and technology. New York: Interscience Publishers, Inc.
Covey, A. D. (1905). The secrets of specialists (2nd. ed.). Detroit: Physicians Supply Company.
deNavarre, M. G. (1941). The chemistry and manufacture of cosmetics. Boston: D. Van Nostrand Company.
deNavarre, M. G. (1975). The chemistry and manufacture of cosmetics (2nd ed., Vol IV). Orlando: Continental Press.
Hairdressing. (1913). May. London, England.
Hansen, K. (2007). Hair or bare?: The history of American women and hair removal, 1914-1934. Retrieved March 13, 2014 from http://history.barnard.edu/sites/default/files/inline/kirstenhansenthesis.pdf
Hilfer, H. (1949). Depilatories. Drug and Cosmetic Industry, 64(2), 164-165.
Hope, C. (1982). Caucasian female body hair and American culture. Journal of American Culture, 5(1), 93-99.
Indiana State Board of Health. (1917). Eleventh annual report of the chemical division of the laboratory of hygiene. Fort Wayne, Ind: Fort Wayne Printing Company.
Kallett, A., & Schlink, F. J. (1933). 1000,000,000 guinea pigs. Dangers in everyday foods, drugs, and cosmetics. New York: Vanguard Press.
Koeune, A. E. (1937). Fundamentals of depilatory formulation & manufacture. Manufacturing Perfumer, February, 158-159.
Koeune, A. E. (1937). Fundamentals of depilatory formulation & manufacture. Part II. Manufacturing Perfumer, April, 15-17.
Lehman, J., & Gaffney, L. (1932). Thallium poisoning: A report of three cases. Annals of Internal Medicine, 6(1), 60-64.
Lloyd, E. (1907). The skin. Its care and treatment (3rd. ed.). Chicago: McIntosh Battery and Optical Company.
McDonough, E. G. (1937). Truth about cosmetics. New York: The Drug and Cosmetics Industry.
Piesse, G. W. S. (1862). The art of perfumery, and the methods of obtaining the odours of plants, with instructions for the manufacture of perfumes for the handkerchief, scented powders, odorous vinegars, dentifrices, pomatums, cosmetics, perfumed soap, etc. To which is added an appendix on preparing artificial fruit-essences, etc (3rd ed.). London: Longman, Green, Longman and Roberts.
Poucher, W. A. (1927). Origin, history and status of depilatories. American Perfumer & Essential Oil Review, February, 661-662.
Reiger, M. M., & Brechner, S. (1975). Depilatories. In M. G. deNavarre. The chemistry and manufacture of cosmetics (2nd ed., Vol IV, pp. 1229-1273). Orlando, Fl: Continental Press.
Reiger, M. M. (2000). Harry’s cosmeticology (8th ed.). Boston, Ma: Chemical Publishing Co., Inc.
The ugly girl papers. (1874). New York: Harper & Brothers.
Verni, M. (1946). Modern beauty culture (2nd ed.). London: New Era Publishing.

1828 Atkinson’s Depilatory.

1841 Dr. Gouraud’s Depilatory Powder.

1896 Modene. The manufacturer also recommended this to men as a replacement for shaving. “Gentlemen who do not appreciate nature’s gift of a beard, will find a priceless boon in Modene, which does away with shaving. It dissolves and destroys the life principle of the hair, thereby rendering its future growth an utter impossibility and is guaranteed to be as harmless as water to the skin.”

1898 Saca-Pelo from the Newton Manufacturing and Chemical Company. Removing upper lip hair became a major issue for many women.

1898 French Depilatory from the American Toilet Company.

1898 Helen E. Marko.

1901 Dr. Rhodes New Hair Remover.

1902 Hair Destroyer from the Beyara Company. This is listed as a ‘Great Syrian Remedy’ so it is possible that it was rhusma.

1905 Maji from the Turkish Remedy Company. Another possible rhusma depilatory.
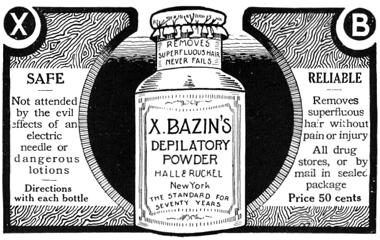
1911 X-Bazin Depilatory Powder from Hall & Ruckel although the bottle lists lists as ‘the standard for seventy years’, that applies to the company not the powder.

1917 Foraline from the Foral Products Company.

1917 Decoltene from A. B. Robartes, Ltd., London.

1918 Delatone from the Sheffield Pharmacal Company.

1919 Evans’s Depilatory.

1921 Flick from the Pan-American Pharmaceutical Company.

1921 Neet from the Hannibal Pharmacal Company.

1922 DeMiracle.

1922 Mi-Rita Superfluous Hair Remover from Dr. Margaret Ruppert..

1923 Mahler treatment for superfluous hair by the D. J. Mahler Company.

1923 Kilrute from the Kilrute Company.

1924 Zip by Madame Berthé.

1926 Del-A-Tone powder and cream depilatory from the Delatone Company

1928 Neet. The ‘inventor’ of this product is given as Rolla Cecil Lawry. However, the original Neet was developed by Edward Druin Frier [1886-1926] in St Louis. Presumably Lawry gained control of the company after Frier’s death. The product, originally called Marvel, was renamed as Neet in 1918. It was called Veet in the U.K. and elsewhere from 1922, a name that would be universally adopted after 2002.

1931 Zip Epilator (wax) and Zip Depilatory Cream.

1931 Snow from the Rasofix Corporation. The product was a depilatory powder. It came with a free fibre brush and was also recommended for men.

1931 Koremlu. Women who used the cream were, for the most part, applying it as an accessory to prevent regrowth after having their hair removed by hot wax.

1938 Veet. The U.K. version of Neet. The Veet name would be adopted in America at a later date.

1941 Imra by Artra Cosmetics, a division of the Schering Corporation. They licensed the thioglycolic depilatory patent from Sales Affiliates.

1949 Nair. Short for No-hair, this depilatory was a lotion not a cream.

1971 Shavine powder.
