Pearl Essence
The increased use of pastel shades in make-up after the Second World War was followed by a fashion for pearl lustre. Previously used only in nail polishes, artificial pearl was introduced into a wide range of cosmetics including make-up, skin creams and hair care products.
Lustre
Cosmetics with a luster have a gentle sheen or soft glow. In some cosmetics it was sought-after, in others it was to be avoided; in eye shadow, luster is very important, whereas in mascara it is more understated. Materials used in cosmetics can be selected on their ability increase or decrease the luster of the final product; matt face powders for example, can include materials like kaolin, calcium carbonate and zinc oxide to help reduce the natural lustre generated by talc.
For many cosmetics luster is synonymous with the shine generated by the reflection of light. In some cosmetics, light is also refracted, and it is the refracted light that produces pearlescent effects. The best example of the visual contrast between the effect of reflected and refracted light in cosmetics is to look at the difference between high quality cold and vanishing creams.
In the case of cold cream, a luster is produced by the emulsion created when the cream is made. Small droplets in the emulsion disperse light producing a lustrous sheen that makes the cream look very attractive in the jar. Better cold cream emulsions have smaller droplets and produce a better sheen but there is no pearly effect. In comparison, a high quality vanishing cream has a lustrous sheen with pearly effects. The reason for this pearlescent effect puzzled cosmetic chemists for many years, as did their inability to generate it in some formulations.
Pearlescent sheen
In the case of natural pearls the pearlescent lustre is generated by light being reflected and refracted through minuscule platelet-shaped crystals made of aragonite (a form of calcium carbonate) in the pearl nacre. These colour effects vary with the angle of the viewer, resulting in the characteristic pearly lustre.
Other substances that have a pearl-like sheen often contain similar platelet-shaped crystals. The effect cosmetic chemists strived to reproduce in vanishing creams was eventually also shown to be the result of crystals generated during manufacture.
See also: Vanishing Creams
Faux pearls
In the seventeenth century it was discovered that an extract from fish scales could be used to make imitation pearls. First used in France using the scales of the bleak fish – a type of carp, Alburnus alburnus, known in France as an ablette – the extract was used to make faux pearls for rosaries at a fraction of the cost of the real thing. Although the visual effects were not identical to natural pearl they were similar enough to deceive the uneducated eye, and before long faux pearls made from fish scale extract were being manufactured throughout Europe.
The importance of the extract can be seen in the variety of names under which it was known – essence d’orient, vermis d’orient, écailles d’ablettes, or just orient in French; fish-scale essence, mother of pearl essence, orient pearl base, essence of orient, pearl essence or Levantine oil in English; and, fischsilber, silber, perl, perlmutterglanz, fischschluppenessenz, and orientalische essenz in German (after Sabetay & Hunsdiecker, 1961).
Non European countries such as Japan and the United States also established industries to produce pearl essence. In the case of the United States this only began seriously in the 1920s, after European supplies had been cut off in the First World War. By this time, pearl essence was used for other things as well; for example, early plastics like celluloid used it to make imitation mother-of-pearl items like costume jewellery, hand mirrors, manicure sets, pearl handles and buttons.
Mother-of-pearl effect
Long after it was first used, essence d’orient or pearl essence was also shown to contain platelet-shaped crystals, in this case made of two purines, guanine and hypoxanthine. It was the light reflecting and refracting through these crystals that produced the mother-of-pearl effect. The ratio of the two crystals and their size varied between fish species with the best quality pigment being obtained from cold-water fish that have larger platelet-shaped crystals.
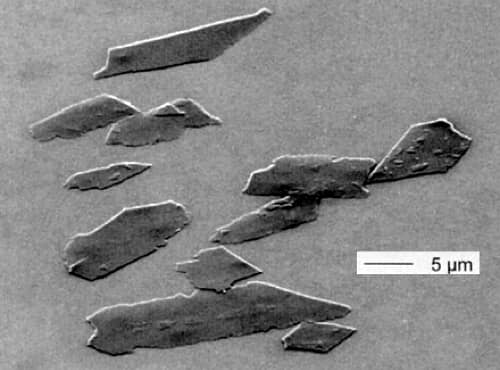
Above: An electron microscope image of the platelet-like crystals found in pearl essence (modified from Pfaff, 2008).
Typically, the pearl essence was obtained as a by-product of the fishing industry. However, as the amount extracted is less than 1% of the guanine and hypoxanthine present in the fish scales, the price of the extract was rather high when compared to other ingredients. Nevertheless, its brilliant lustre and lack of toxicity made it a good fit for some cosmetics.
Nail polishes
The earliest commercial use of pearl essence in cosmetics was in nail polishes in the 1920s. If made for this use the pearl essence would be dispersed in nitrocellulose-amyl or butyl acetate, with the concentrate making up between 4-8% of the final nail polish mixture. The amount used depended on the level of lustre desired, the amount of colour and the opacity of the polish. More additives required larger amounts of pearl essence to achieve a good lustre.
One problem with using pearl essence in nail polishes was its tendency to sink to the bottom of the bottle – known as sedimentation – an issue shared with other suspended particles as well. Rather than add a ‘Shake Before Use’ label, chemists employed a variety of chemical tricks to reduce the problem but often had to resort to substituting some of the better quality pearl essence with lower grade material from Japan. The Japanese pearl essence did not have as good a lustre but its guanine and hypoxanthine crystals were more needle-like which gave them the advantage of slow sedimentation, i. e., they were less likely to settle out (Hibbott, 1963).
See also: Nail Polish
Other cosmetics
After first being used in nail polishes, pearl essence found its way into other make-up products like lipsticks, eyeshadow and eyeliners. Some cosmetic chemists also added it to foundations and other creams. Depending on the cosmetic in which it was to be used, the pearl essence was stored and distributed in different liquids. For example, if it was to be used in lipstick it could be suspended in castor oil; whereas, if it was to be put into a face cream, it could be suspended in petrolatum, fat or oil. As with nail polishes, the amount used depended on a range of factors – the effect required, the medium, the amount of colouring and the opacity the product. Cosmetics that contained large proportions of coloured and opaque pigments, such as lipsticks, required higher levels of pearl essence, where as transparent creams – that only needed a subtle lustre – contained less.
Other pearlescent pigments
As well as its relatively high cost, pearl essence had another limitation. It was not possible to produce it in a dry form because the crystals would clump. This meant it could not be used in face or other powders. To add lustre to these products – and to find cheaper ways of introducing mother-of-pearl effects into cosmetics – chemists turned to other materials.
Bismuth oxychloride: Bismuth oxychloride had been used in face powders in the nineteenth century as one of the ‘bismuth whites’ and powder made with it was sometimes referred to by names such as pearl powder, pearl white, or blanc de perles, perhaps in part because of its slight pearly sheen.
See also: Pearl Powders.
When first used in cosmetics, bismuth oxychloride was generally incorporated as a filler, due to its good adhesive qualities and smooth feel on the skin. The substance was first sourced from mineral deposits but in the 1930s it began to be produced chemically in laboratories. By carefully controlling the chemical reactions that produced it, chemists made forms that had larger crystals with an enhanced pearlescence, thereby producing the world’s first synthetic, non-toxic, pearl lustre.
As well as being available in a dry state, bismuth oxychloride could be made in stronger concentrations than pearl essence and at a cheaper price, which made it doubly attractive. Although not as effective as native pearl essence, it reduced the cost of making pearly shaded nail polishes, lipsticks, eyeshadows and so forth, and also enabled pearlescent effects to be added to face powders and other cosmetics.
Mica: Although the pearlescent effect produced by coating mica with titanium dioxide had been described back in 1942 (Pfaff, 2008) it was not until the 1960s that this – and similarly coated mica pigments – became widely available. Natural mica (muscovite) is one of the most abundant minerals on the planet and could be ground to produce platelets of varying sizes. A wide range of colours could be produced by coating the mica with one or more layers of pigment. Different effects could be generated by using mica plates of different sizes; smaller mica platelets would produce a sheen, larger ones glitter. Their introduction into cosmetics in the 1960s can be seen in the Space Age Look and other fashion trends of that decade.
Newer materials: It is beyond the scope of this story to detail the more recent developments in this area – which are numerous and ongoing – except to say that innovation generally ran in two directions. The first was the development of new platelets, including flakes made of aluminium oxide (aluminium), silicon dioxide (silica), borosilicate (glass) and fluorophlogopite (synthetic mica). The second was the incorporation and layering of new pigments on these flakes, in particular the use of mulitlayered pigments. Together these produced a range of pearlescent, lustre and iridescent effects only dreamt of in earlier times.
Updated: 1st March 2016
Sources
Hibbott, H. W. (Ed.). (1963) Handbook of cosmetic science: An introduction to principles and applications New York: The MacMillan Company.
Krajkeman, A. J. (1952). Pearl essence. Manufacturing Chemist. July, pp. 278-281.
Pfaff, G. (2008). Special effect pigments (2nd ed.). Isernhagen: Dobler-Druck.
Sabetay, S. & Hunsdiecker, H. (1961). Nacreous bases in cosmetics. Soaps, Perfumes and Cosmetics. January, pp 57-60.
Schlossman, M. L. (Ed.). (2002). The chemistry and manufacture of cosmetics (3rd ed., Vol. III). Carol Stream, Il: Allured Publishing Corporation.
Taylor, H. F. (1926). Pearl essence: Its history, chemistry and technology. In Report of the United States Commissioner of Fisheries for the Fiscal Year 1925 with Appendixes (pp. 15-36). Washington: Government Printing Office.

1966 Part of an advertisement for Revlon Frostlings. “Pearl dipped pales for lips and nails”.

The bleak fish Alburnus alburnus, known in France as an ablette.

Manufacturing faux pearls in the eighteenth century.

1870 Making faux pearls.

1959 Cyclax Pearlescent lipsticks.

1959 Richard Hudnut Cloudsilk Pearled Face Powder.

1960 Cutex Pearl Polishes

1966 Shiny Slicker Lipsticks from Yardley
