Deodorants and Antiperspirants
Products developed to control underarm wetness and odour generate huge sales volumes today. Underarm wetness and odour have different causes: the wet patches are due to excessive perspiration from eccrine skin glands but the unpleasant odour comes largely from secretions by the apocrine glands after they become active at puberty.
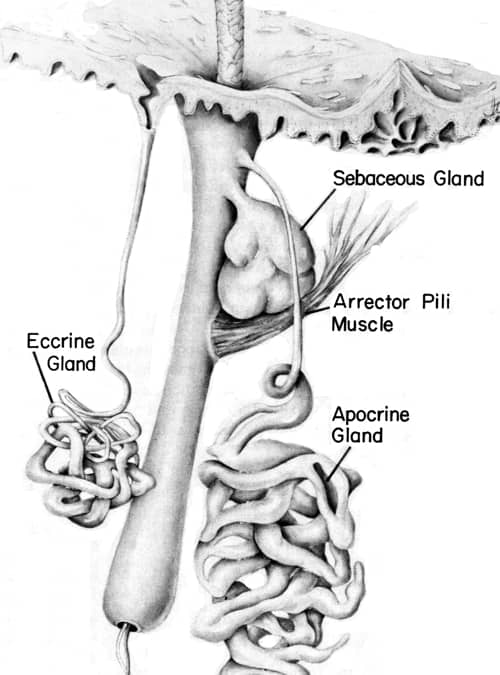
Above: Diagram of the eccrine and apocrine skin glands close to a hair follicle (Montagna & Parakkal, 1974). The distinction between the two glands was first described in 1922 by the German scientist Dr. Paul Schiefferdecker [1849-1931].
Apocrine and eccrine sweat is relatively odourless when produced unless affected by foodstuffs such as garlic. Apocrine sweat contains organic material – cholesterol, sterols, lipids and proteins – that can be broken down by bacteria into acrid-smelling substances. The bad smell is more noticeable in the armpit (axilla) as apocrine glands are very numerous there and underarm hair helps disperse the malodour into the air.
Above: Smell-testing the effectiveness of an underarm deodorant.
In the past, the heavy perspiration and malodour in the armpit might be alleviated by washing the body to remove bacteria and body secretions, wearing undergarments and/or dress shields to limit outer clothing marks, and using perfume to cover body odour. However, as standards of personal hygiene rose in the twentieth century, more and more individuals chose to use products to control the production of underarm sweat and the odour it produced rather than deal with its consequences. Early American examples include: Mum (1888), Amolin (1893), Ever-Dry (1903), Hush (1908), Odorono (1909), and Non-Spi (1910).
The increased demand in twentieth century for these products started in the American market as companies – such as the Mum Manufacturing Company of Philadelphia, makers of Mum deodorant, and the Odorono Company, Cincinnati, makers of Odo-Ro-No deodorant/antiperspirant – conducted extensive advertising campaigns to convince American women – and later men – that they needed to do something about the problem of underarm sweat
Odo-On-No is particularly noteworthy as its advertisements introduced the practice of ‘whisper-copy’ which was used to convince women that they were suffering from a problem that others were too polite to bring to their attention. Whisper-copy would go on to help sell a wide range of personal products.
See also: Odorono
In 1938, it was estimated that 60% of women and 20% of men in the United States used a product to control underarm odour (deodorant) and/or perspiration (antiperspirant). Some of these products were specifically designed just for men, the earliest examples that I know of being Top-Flite and Stymie, both deodorants introduced in 1935. However, going on the newspaper report below, it is likely that there were earlier examples.
Magic passwords such as “Open Sesame” have always fascinated the readers of fairy tales. Modern business men are seeking treasure through a study of the effect of words in inducing people to buy. The Tested Selling Institute is studying the effect of words on thousands of customers of stores, soda fountains and candy shops.
The method is simply one of trial and error, as well as making use of the accumulated experience of hundreds of cases. For Instance, a drug chain attempted to sell a deodorant to men by suggesting to women purchasers that they call it to the attention of their husbands.
The effort was a complete failure. Customers would snap back such remarks as, “What makes you think I have a husband?” or “What makes you think he needs it young woman?” It was then placed at the cigar counter and brought to the direct attention of men. But the question “How about some deodorant today?” was naturally resented.
Finally the deodorant and a cake of soap widely advertised for its deodorant qualities were placed on the counter under a sign reading: “For Men.” Men knew the significance of the soap because it was widely advertised and became immediately interested in the deodorant and asked questions about it. When the sign was finally changed to, “For Active Men,” six out of every ten men who came to the cigar counter stopped and asked about the product.(Eager, 1935)
Controlling underarm sweat
Jellinek (1970) describes six possible methods for controlling underarm sweat and odour:
1. Inhibiting perspiration though the use of drugs that affect the nervous system;
2. Stopping perspiration by blocking the sweat ducts;
3. Removing perspiration after it reaches the skin surface;
4. Inhibiting or killing the bacteria responsible for producing body odour;
5. Destroying or removing the odoriferous substances after they have been developed; and
6. Masking body odour with fragrances.
Antiperspirants work by blocking the sweat ducts (2) whereas most deodorants inhibit or kill the bacteria responsible for producing body odour and/or cover the odour with a fragrance (4, 6). Antiperspirants therefore affect a body function and many countries therefore classify them as over-the-counter (OTC) drugs and regulated them accordingly, legally distinguishing them from deodorants whose effect is merely cosmetic.
The legislative distinction between an antiperspirant and a deodorant began in the United States with the passing of the U.S. Food, Drug & Cosmetic Act (FD&CA) in 1938. Other countries followed at a much later date. This may explain why early cosmetic chemists generally viewed antiperspirants as a type of deodorant and labelled them accordingly.
Deodorants intended for use on the body fall readily into two main types, i.e., those that are meant merely to deodorize the perspiration and those which contain a sufficiently appreciable content of astringents [so] as temporarily to check the flow of perspiration altogether.
(Jannaway, 1935, p. 375)
It is also possible that many American companies continued to label their antiperspirants as deodorants after 1938 so that they were treated as cosmetics, not drugs, by the Food and Drug Administration (FDA).
Antiperspirants
Over the years many theories have been proposed to explain the action of antiperspirants, an early view being that they acted as astringents to close the pores.
The simplest method of inhibiting perspiration is by the use of an astringent which acts partly by coagulating the proteins in the surface of the epidermis and partly on the nerve reflexes by closing the pores, thus achieving the desired result.
(Silman, 1934, p. 323)
Astringent mixtures act by precipitating protein, reversibly or irreversibly, thus partially closing pores and stopping the flow of perspiration at its source.
(Kalish, 1940, p. 410)
Using astringents as antiperspirants was not a new idea. Alum – a naturally-occurring astringent containing aluminium and potassium sulphates – had been used in the West as an antiperspirant from Greek and Roman times. It was also employed by nineteenth and early twentieth century doctors to treat excessive sweating (hyperhidrosis).
Although astringent aluminium salts proved to be the most effective antiperspirants, and were widely used, cosmetic chemists examined a wide range of substances for similar capabilities.
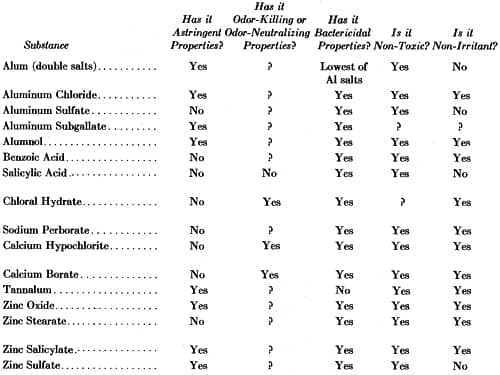
Above: Comparison of Deodorant Substances (Weekes, 1933).
Aluminium salts
In 1916, the ability of aluminium salts to control perspiration received scientific support when Dr. Arthur William Stillians [1871-1967] reported that aluminium hexachloride could be used to control localised hyperhidrosis. However, the first aluminium salt to be used in a commercial antiperspirant was aluminium chloride.
Ever-Dry (1903) – now generally regarded as the first commercial antiperspirant – and Hush (1908) both used aluminium chloride as the active ingredient (Laden & Felger, 1988, p. 2). How Ever-Dry came up with the idea of using aluminium chloride is a mystery but it has been suggested that late nineteenth-century actors and actresses experimented with aluminium chloride to reduce perspiration when working in heavy costumes under hot electric lights (Klepak & Walkey, 2000, p. 70), so the idea may have come from them. However, aluminium chloride was widely used in the nineteenth century as an external disinfectant. Chloralum for example – made with aluminium chloride – was a well-known disinfectant and numerous formulas for it were published. So, it is possible that Ever-Dry selected to use aluminium chloride as a deodorant rather than an antiperspirant.
Most of the early antiperspirants were made with aluminium salts, with aluminium chloride, aluminium sulphate and alum being most commonly employed. If scented, diethylene glycol ethyl ether could be added to help incorporate the perfume into the mixture, giving the final product some additional odour-covering capability.
Per Cent Alum 20.0 Water 76.5 Ethyl ether of diethylene glycol 3.0 Perfume 0.5 (Chilson, 1934, p. 170)
Aluminium chloride was generally regarded as the most effective antiperspirant in the 1930s but personal-care products made with it were relatively acidic and were liable to irritate the skin and rot clothing fabric. Two solutions to this problem were to use less acidic aluminium salts such as aluminium sulphate and/or to buffer the antiperspirant with agents such as urea or borax. Both of these fixes made the antiperspirant less acidic but the trade-off was that they also made it less effective.
Aluminium chloride 90 Aluminium sulphate 40 Borax 10 Water 860 1000 (Jannaway, 1935, p. 375)
Over time, investigations by cosmetic chemists led to the introduction of other aluminium salts. In the long run there was widespread industry adoption of aluminium chlorohydrate (ACH), first introduced in 1947. Initially controlled by a patent (US2,492,085, 1949), invalidated in 1954, it was less acidic that other aluminium salts so was less likely to irritate the skin. It was also kinder to fabrics and a good antibacterial.
Clothing stains continued to be long-term issue. Alkaline soaps and detergents reacted with aluminium salts to generate yellow stains which could be set by ironing the fabric between washes. If the aluminium salt was contaminated with iron the problem could be even worse.
Zirconium salts
Zirconium salts were introduced in the 1950s. Early forms gave way to zirconium chlorohydrate with modern formulations commonly using aluminium zirconium chlorohydrate (AZCH). This has a higher antiperspirant efficacy than aluminium chlorohydrate (Kilpatrick et al., 2009, p. 1002), although the later is still used in some modern-day antiperspirants.
Deodorants
Toilet waters had been used for centuries to mask body odour. Some of these – such as the traditional Eau de Cologne – had some antiseptic value that helped to curb the activity of the Corynebacteria that are the main cause underarm odour. Scented talcum powders were another popular deodoriser. The perfume helped cover odour, talc absorbed some of the underarm perspiration, zinc or magnesium stearate helped the powder adhere, and there was a selection of other ingredients – such as zinc peroxide, boric acid and sodium perborate – that acted as deodorisers. A simple recipe for a talcum powder is listed below:
Per Cent Purified zinc peroxide 40.0 Boric acid 20.0 Talc 39.5 Perfume 0.5 (Chilson, 1934, p. 177)
During the second half of the twentieth century a number of modern antibacterial compounds were introduced into deodorants. Only some of these will be covered here.
Antibiotics
The astonishing success of penicillin in controlling infection during the Second World War generated a massive post-war interest in antibiotics. In 1950s, antibiotics found their way into a range of consumer products including deodorants; the most commonly employed antibiotics being neomycin and tyrothricin. Their use declined in the 1960s as the concern over the indiscriminate use of antibiotics led companies to switch to other antibacterials such as hexachlorophene.
See also: Antibiotics in Cosmetics
Chlorophyll
In 1950, Dr. F. Howard Westcott [1902-1989] described the potential use of chlorophyll and chlorophyllins as mouth and body deodorisers. Subsequently, chlorophyll derivatives found their way into toothpastes and deodorants as well as mints, chewing gums and deodorising pills.

Above: 1950 Nullo Chlorophyll Tablets taken to reduce body odour and bad breath.
However, interest in them declined when it was realised that they were ineffective at concentrations low enough to avoid having the chlorophyll stain clothing.
Hexachlorophene
Hexachlorophene was first suggested as an antiseptic in the 1930s but it was not until it was incorporated into Dial Soap in 1948 that it began to receive widespread attention from the cosmetic industry.

Above: 1952 Cidal Germicidal Soap, one of many products to use hexachlorophene.
Hexachlorophene – also known as G-11 and a variety of other generic names – was used a number of deodorants but was abandoned in the 1970s when it was found to adversely affect the nervous system.
See also: Hexachlorophene
Triclosan
Triclosan was developed by the Swiss company, Ciba-Geigy in the 1960s and found its first use in hospital scrubs in the 1970s. Many cosmetic companies began using it when problems with hexachlorophene became evident and it was employed as an antibacterial in a wide variety of personal products including soaps, shampoos, deodorants, toothpastes and mouthwashes. Concerns about its long-term effect on health and the environment have seen its use decline in recent years.
Product forms
Underarm deodorants and antiperspirants have been dispensed in a wide variety of ways including powders, liquids, creams, sticks, squeeze bottles, roll-ons, aerosols and pump packs, with different forms becoming more or less popular over time.
Competition between companies manufacturing these lines was fierce. Developing a new way to deliver an antiperspirant or deodorant was often accompanied by a large increase in sales and the first entrant often became the leading product for many years to come.
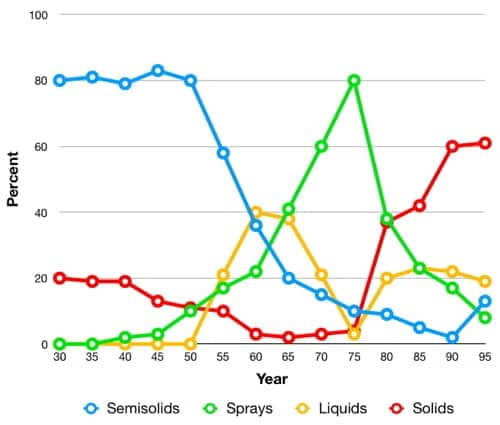
Above: American deodorants and antiperspirant product forms over time in the U.S. market (modified from Giovanniello, 1996).
Early deodorants came as liquids, powders, pastes, compacts and sticks but early antiperspirants were generally sold as liquids or pastes as this would enable them to readily penetrate into the sweat ducts.
Powders
As mentioned earlier, powders were a common form of deodorant and their use persisted through the twentieth century. Most were formulated for general use rather than specifically for the armpit. These were usually packaged in tins with a perforated lid – later replaced with plastics – but there were also forms packaged much like face powders with a puff that could be used on specific body areas.

Above: Spiro Deodorant Powder with puff. The product contained boric acid, magnesium carbonate and zinc oxide.
As with face powders, compressed deodorant powders were also made. A sample recipe is given below:
Per Cent Boric acid 20.0 Talc 40.0 Rice starch 5.0 Cocoa butter 5.0 Oxyquinolin sulfate 5.0 Tragacanth mucilage 5% 18.0 Titanium dioxide 6.0 Perfume 1.0 Procedure: Dissolve the oxyquinolin in the tragacanth mucilage; stir in the starch thoroughly, melt and add the cocoa butter. Mix and sift the talc, boric acid, and titanium dioxide, and add binder. Add the perfume. Mix until a very stiff dough is made. Compress into blocks and allow to dry.
(Chilson, 1934, pp. 175-176)
Although not very popular, compressing the powder meant that it was less likely to spill, making it safer when carried in a purse. It was also possible to included modern deodorisers into a powdered deodorant. The recipe below uses hexachlorophene:
Per Cent Talc 84.0 Boric acid 3.0 Chalk 12.0 Cetyl alcohol 0.5 Hexachlorophene 0.5 Perfume q.s. Procedure: Dissolve the hexachlorophene and cetyl alcohol in a minimum quantity of alcohol and add to the dry powders.
(Plechner, 1957, p. 732)
Liquids
Perfumes and toilet waters used to cover body odour were liquids but leaving them aside, most of the early liquid personal products were antiperspirants. Despite the fact that they were generally thin and drippy, they were the only form of commercial antiperspirant available until the 1930s.

Above: An early image of Nonspi, a liquid antiperspirant introduced in 1910. The bottle is sealed with a cork covered with wax to prevent evaporation.
Glass containers were used to avoid corrosion from the aluminium salts and a good seal was required to reduce evaporation. Early versions used corks to seal the bottle but these were later replaced with plastic screw top lids. The liquid needed a cloth or sponge to apply it but later forms often included a convenient applicator built into the lid, although this might come with a higher price point.

Above: Applying Check Deodorant with a sponge applicator.
Some manufacturers investigated the possibility of delivering the liquid using something akin to a perfume atomiser. However, the acidic nature of the antiperspirant made the development of such a device difficult even after the introduction of plastics. Despite these problems the idea was a good one as the later success of the polyethylene squeeze bottle made evident.
Pastes and creams
Pastes were the preferred medium for early deodorants. As well as containing a deodoriser – such as zinc oxide or zinc peroxide – most were made with a base of petrolatum, lard or some other fat. The ability of fat to absorb odours was well known to perfume makers who used it to capture flower fragrances, a process known as enfleurage. The recipe below is for a petrolatum-based paste:
Zinc stearate 4.00 Zinc oxide 20.00 Glycerine 30.00 Corn starch 12.00 Petrolatum 33.75 Balsam Peru 0.20 Oil cassia 0.05 100.00 (Auch, 1937, p. 281)
As well as pastes, deodorant creams were also made using a base formulated like a beeswax/borax cold cream.
White wax 8 oz. Liquid petrolatum 24 oz. Borax 100 gr. Benzoic acid 20 gr. Salicylic acid 400 gr. Hot water 6 oz. The wax is first melted and heated to 160°F. The other materials are dissolved in the water and heated to the same temperature; the two are then mixed and a beautiful glossy cream results.
(Silman, 1934, p. 324)
These were later superseded by deodorant creams made with a vanishing cream base. These felt less greasy and, if the cream was rubbed well in, or the armpit was wiped with a damp cloth before dressing, had a better feel and were less likely to leave marks on clothing. The formula below uses methenamine as an antiseptic, more commonly used as a deodorant for the foot than the underarm:
Parts Stearic acid 20.0 Potassium hydroxide 0.8 Sodium hydroxide 0.2 Glycerine 5.0 Methenamine 5.0 Water 67.0 Titanium oxide 2.0 Perfume qs. (Maicki, 1938, p. 36)
In 1936, Feminine Products – a division of Carter Medicine – introduced Arrid, the first commercially successful antiperspirant cream containing aluminium sulphate. It was easy to apply and dried quickly so, although it was less effective than liquid aluminium chloride antiperspirants, it became very popular, capturing over 34% of the American antiperspirant-deodorant market by 1945 (Laden & Felger, 1988, p. 4). Its success led to creams dominating this market for the next twenty years.

Above: 1938 English advertisement for Arrid Cream Deodorant.
Sticks
Early stick deodorants were created using a lipstick base.
Per Cent Beeswax 34 Cocoa butter 8 Lanolin 6 Petrolatum 18 Paraffin wax 10 Oxyquinolin sulfate 3 Zinc salicylate 2 Zinc sulfocarbonate 2 Talc 16 Perfume 1 Procedure: Mix the oxyquinolin sulfate, zinc salicylate and sulfocarbonate with the petrolatum and grind through an ointment mill until the mass is smooth. Melt the beeswax, cocoa butter, paraffin and lanolin and add the petrolatum base; mix thoroughly and slowly then sift in the talc while mixing. Add the perfume and cast into sticks.
(Chilson, 1934, p. 176)
Later versions were made by gelling propylene glycol and/or alcohol with sodium stearate in a manner similar to manufacturing a cologne stick. These were a big improvement on earlier forms but evaporation problems meant that the sticks had to be encased in a container with a good seal.

Above: 1956 Fresh Stick Deodorant. To reduce evaporation, the stick was stored in the glass container with a well-sealed screw-top lid.
In the formula below chlorhexidine diacetate is used as the bactericide:
Sodium stearate 70 Cetyl alcohol 15 Propylene glycol 50 Alcohol 830 Chlorhexidine diacetate 5 Water (softened or distilled) 30 1000 Perfume 0.5 percent Procedure: Heat all the materials together under total reflux until all the soap is dissolved. Colour solution if required can be added. The temperature is allowed to fall to 65°C and perfume added to the cooling base. Mix well and transfer to suitable moulds.
(Poucher, 1974, p. 19)
As these sticks were alkaline it was necessary to buffer them before adding an antiperspirant, e.g., aluminium chlorohydrate (ACH) could be buffered with sodium lactate to make sodium aluminium chlorohydroxylactate. The antiperspirant effect of these buffered aluminium salts was low so the sticks often incorporated a deodorant as well. In the 1960s, more effective stick antiperspirants were developed using aluminium chlorohydrate propylene glycol. Consumer interest in these products declined after aerosol antiperspirants were introduced but revived in the late 1970s after sales of aerosols crashed and new anhydrous stick deodorant/antiperspirants were developed. These used volatile silicones such as cyclomethicone which quickly evaporated after they were applied leaving the skin feeling smooth and dry.
Squeeze bottles
The first squeeze bottle spray deodorant/antiperspirant was Stopette (1947), developed by Jules Montenier [1895-1962]. The squeezable container was made from blow-moulded polyethylene, selected because it was unaffected by aluminium chloride.
See also: Stopette
Although it avoided direct contact between the applicator and the skin, the spray required a low viscosity formula which felt wet, was slow drying and could drip down the arms and body. Despite these limitations Stopette was very popular and other companies quickly adopted this type of dispenser.

Above: Mr. Fresh Spray Deodorant.
Like the earlier creams and liquids their popularity declined with the introduction of roll-ons and aerosols.
Roll-ons
Roll-ons made their appearance in 1952 with the introduction of Mum Rollette from Bristol-Myers. The product had delivery problems and was withdrawn from sale but its successor, Ban, released in 1955, went on to become the third highest-selling deodorant in the United States and was widely copied.
See also: Ban
Over the years, the liquid deodorant/antiperspirants used in roll-ons came in range of formulations including emulsions, gels and suspensions. Like sticks, their popularity declined and rebounded with the rise and fall of aerosols in the 1960s and 1970s and the development of new roll-on formulations using volatile silicones, such as Dry Idea, released by Gillette in 1978.
Aerosols
The aerosol dispenser was developed during the Second World War to spray insecticides. After the war they were used to deliver a range of cosmetics and other personal products. Cosmetic chemists faced a number of technical problems when developing an aerosol antiperspirant or deodorant including corrosion of the metal containers, clogged valves and incompatibilities with perfumes, so it was a number of years before successful products were developed.
The first aerosol deodorant/antiperspirant that was commercially successful was Right Guard, released by Gillette in 1960 using zinc phenolsulphonate as an antiperspirant and hexachlorophene as a deodorant. Later testing revealed that zinc phenolsulphonate did not appreciably reduce underarm sweating so it was dropped from the formulation along with any antiperspirant claims for the product (Laden & Felger, 1988, p. 6). The high levels of alcohol in the formula meant that it was quick drying and, because the nozzle did not touch the skin, it could be used by the entire family, something that became a major selling point for all the deodorant and antiperspirant aerosols that followed.

Above: 1973 a range of aerosol deodorants and antiperspirants.
In 1967, Carter-Wallace introduced Arrid Extra Dry, the first commercially successful aerosol antiperspirant. It solved the corrosion and valve-clogging problems by suspending the aluminium salt in an oil base. As the powder-in-oil formulation did not contain water this prevented corrosion of the metal container and the build up of valve-blocking crystals. It was an instant success and dominated the market for years to come.
See also: Aerosol Cosmetics
Extensive research into antiperspirant and deodorant aerosols followed and a number of new formulations were developed. These led to the domination of aerosols in the antiperspirant-deodorant market until the mid 1970s when safety concerns and the banning of chlorofluorinated hydrocarbon (CFC) propellants – which were shown to be damaging the ozone layer – led to a dramatic reduction in sales and the resurgence of roll-ons and sticks and a greater use of pump packs. Over time, with the replacement of CFCs by hydrochlorofluorocarbons (HCFCs) and ultimately hydrofluorocarbons (HFCs), public confidence in aerosols returned and aerosols recaptured a significant part of the deodorant-antiperspirant market which they still hold today.
Health issues
Health concerns about antiperspirants and deodorants started when antiperspirants were first introduced in the early twentieth century. At the time, the medical profession were against deploying aluminium salts in mass-marketed products as they irritated the skin and were thought to interfere with the healthy action of the pores. However, by the 1930s this medical opposition had largely died away.
In those days there was active resistance to their use by doctors, nurses and public health officials. …
This has all changed to-day and medical journals frequently contain articles, clinical evidence and all, tending to prove (what we have known for over two decades) that bromidrosis (odorous or foetid sweat) and hyperhidrosis (excessive perspiration) have been harmlessly and effectively corrected.(Auch, 1937, p. 279)
Later health concerns were mostly due to the chemicals introduced into deodorants and antiperspirants after the Second World War. Hexachlorophene was banned when it was discovered to be a neurotoxin; the use of zirconium salts in aerosols was discontinued after they were suspected of being carcinogenic when inhaled; and the use of triclosan has declined due to a range of health and environmental concerns.
See also: Hexachlorophene
In general, despite claims to the contrary, products currently on the market are safe to use if applied correctly. Some countries – such as Australia – have even altered their regulations to classify antiperspirants as cosmetics thereby reducing the level of control on these personal products.
See also: What is a Cosmetic?
First Posted: 27th June 2017
Last Update: 11th August 2023
Sources
Auch, R. H. (1937). Under-arm deodorants. Manufacturing Perfumer, December, 279-281.
Bien, R. R. (1946). Action of antiperspirant creams on fabrics. The American Perfumer & Essential Oil Review. January, 47-50.
Chilson, F. (1934). Modern cosmetics. New York: Drug & Cosmetic Industry.
Conry, T. (1980). Consumer’s guide to cosmetics. Garden City, NY: Anchor Press.
Eager, G. T. (1935). Dollar makers. The Binghamton Press, September 6, p. 31
Food and Drug Administration. (1978). Antiperspirant drug products for over the counter use. Federal Register October 10, Part III, 46694-46732. Washington, DC: Office of the Federal Register.
Giovanniello, R. (1966). Antiperspirants and deodorants. In D. F. Williams, & W. H. Schmitt, (Eds.). Chemistry and technology of the cosmetics and toiletries industry (2nd ed., pp. 310-343). London: Blackie Academic & Professional.
Inaba, M., & Inaba, Y. (1992). Human body odor: Etiology, treatment, and related factors. Tokyo: Springer-Verlag.
Hyde, J. N. (1888). A practical treatise on diseases of the skin, for the use of students and practitioners (2nd ed.). Philadelphia, PA: Lea Brother & Co.
Jannaway, S. P. (1935). Improved deodorants. The Perfumery and Essential Oil Record, October, 375-377.
Jellinek, J. S. (1970). Formulation and function of cosmetics (G. L. Fenton, Trans.). New York: Wiley-Interscience.
Kalish, J. (1940). Cosmetic manual. The Drug and Cosmetic Industry. 48(4), 410-411, 419.
Kilpatrick, L., Misner, S., Recchia, R., Pan, l., Fan, A., & Callaghan, D. T. (2009). Antiperspirants and deodorants. In M. L. Schlossman, (Ed.). (2009). The chemistry and manufacture of cosmetics (4th ed., Vol. 2, pp. 993-1039). Carol Stream, IL: Allured Publishing Corporation.
Klarmann, E. G. (1948). The cosmetic aspects of perspiration and its control. The American Perfumer & Essential Oil Review. July, 33-40.
Klarmann, E. G. (1962). Cosmetic chemistry for dermatologists. Springfield, IL: Charles C. Thomas.
Klepak, P., & Walkey, J. (2000). Antiperspirants and deodorants. In H. Butler, (Ed.). Poucher’s perfumes, cosmetics and soaps (10th ed., pp. 69-100). London: Kluwer Academic Publishers.
Laden, K., & Felger, C. B. (Eds.) (1988). Antiperspirants and deodorants. New York: Marcel Dekker, Inc.
Maicki, R. J. (1938). The newer deodorant creams. The American Perfumer & Essential Oil Review. May, 35-36.
Montagna, W. (1963). The anatomy of sweat glands. Journal of the Society of Cosmetic Chemists, 14(12), 641-652.
Montagna, W., & Parakkal, P. (1974). The structure and function of skin (3rd ed.). New York: Academic Press.
Plechner, S. I. (1957). Antiperspirants and deodorants. In E. Sagarin, E. (Ed.). Cosmetics: Science and technology (pp. 717-739). New York: Interscience Publishers, Inc.
Poucher, W. A. (1974). Perfumes, cosmetics and soaps (8th ed., Vol. 3). London: Chapman & Hall Ltd.
Silman, H. (1934). Deodorants in theory and practice. The Manufacturing chemist, October, 323-324, 327.
Stillians, A. W. (1916). The control of localized hyperhidrosis. The Journal of the American Medical Association, LXVII(27), 2015-2016.
Weekes, C. (1933). Body deodorants. The Drug and Cosmetic Industry. 32(3), 215-219.
Wells, F. V., & Lubowe, I. I. (1964). Cosmetics and the skin. New York: Reinhold Publishing Corporation.

1898 A deodorant from E. L. Pieck.

1904 Coolene Lotion, probably an antiperspirant.
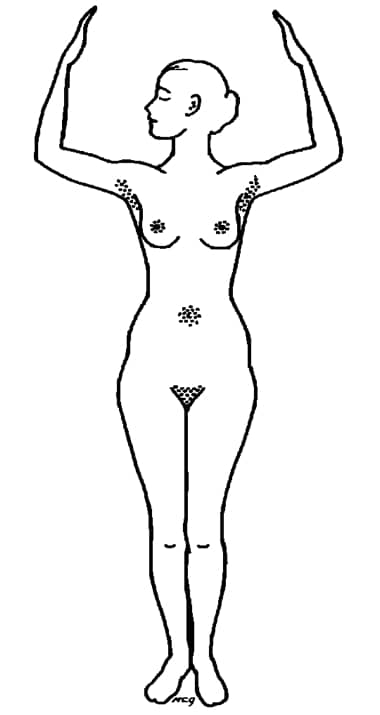
The major sites of apocrine sweat glands on the human body (Montagna, 1963). Apocrine glands only become active at puberty – which is why children are generally sweet smelling.

1905 Spiro Deodorant Powder. Note the woman on the right using a puff to apply it to her underarm.

1907 No-O-Dor Powder. This deodorant could be applied to clothing as well as the skin.

1910 Drosis Antiseptic Deodorant Toilet Powder.

1915 Dry-Pits Lotion.

1915 Colgate Talc Powder.

1918 Odo-Ro-No.

1919 Mum, a cream deodorant.

1920 Eversweet, a cream deodorant.

1923 Ab-Scent, a deodorant and antiperspirant lotion.

1926 Deodo, a deodorant powder.

1935 Shun, a deodorant cream.

1936 Perstik, an early stick deodorant in a case designed like a lipstick.

1936 Perstop.

1937 Nonspi, a liquid deodorant and antiperspirant.

1937 Hush Cream, Instant Hush Liquid Deodorant, Hush Stick Deodorant and Personal Deodorant Powder for sanitary pads.

1945 Lor-Odo, a deodorant stick.

1947 Barbasol Lotion Deodorant.

1948 Veto Cream Deodorant.

1948 Dryad Cream Deodorant.

1950 Odo-Ro-No Cream.

1950 Coty Shakti Deodorant Powder in a squeeze pack.

1950 5-Day Deodorant Pads.

1952 Mennen Spray Deodorant for Men in a squeeze pack.

1954 Mum Cream Deodorant with M-3 (hexachlorophene).

1956 Lander Stick Deodorant with chlorophyll. Note the glass container and tight seal. The product would have been removed from the container before it was used.

1956 Ban, the first commercially successful roll-on antiperspirant.

1957 Sportsman D-Bar Solidified Deodorant.

1959 Odo-Ro-No Stick Deodorant (Germany).

1959 Tussy Cream, Stick and Roll-on Deodorant.

1961 Bac Spray Deodorant (Germany).

1962 Printil Flacon-Bille (Roll-On), Stick and Atomiseur (Pump Pack) Deodorant (France). The description suggests it was also an antiperspirant.

1962 Check Stick, Roll-On and Spray (squeeze pack).

1962 Mennen Speed Stick Deodorant. By the 1960s many stick deodorants were getting larger.

1968 Gilette Right Guard Aerosol Antiperspirant.

1974 Sure Aerosol Antiperspirant.
