Hormone Creams, Oils and Serums
Late in 1931, Rubinstein announced to her American clients that she had just returned from Paris with a new youthifying treatment. She extended an invitation to them to come to her New York salon where she would advise them on the use of her Hormone Twin Youthifiers, a day cream (Twin 1) and a night cream (Twin 2).
Two unique, biological creams—the Hormone Twins supply in assimilable form, actual youth-hormones: stimulators and rebuilders of new, young skin cells.
The most amazing results in recreated beauty are being achieved with these dual creams. For lines, wrinkles and weather-beaten, aging skins, the Hormone Twin Youthifiers give the most gratifying results, building up starved, undernourished tissues, correcting wrinkles, crowsfeet, sallowness, revitalizing the tissues.
For younger skins—prematurely worn, suffering from the tension of nerve strain and fatigue the Hormone Twin Youthifiers prove a quick pick-me-up, overcoming minor flaws and keeping the skin vibrant with youth, exquisitely clear, always at the peak of its youthful perfection!
Each cream may be used alone—the Day Cream as a quick rejuvenating treatment, the Night Cream or Feeder to be absorbed deep into the skin layers, stimulating the processes by which the skin renews its youth.
Together, both creams work in undisturbed, united harmony—reviving the beauty of your skin, correcting every flaw, developing new and lasting loveliness.(Helena Rubinstein, 1932, pp. 1-2)
Also see the company booklet: The Hormone Twins
It might be thought that Rubinstein’s Hormone Twins contained oestrogenic hormones but this may not be the case. First, only the Night Cream appears to have included the rejuvenating ingredient.
If your skin is very dull, lined or dry, Helena rubinstein’s marvelous biological creams—the Hormone Twin Youthifiers, will replace the vital elements of youth. The Hormone Creams consist of Day Cream—which prepares the skin for—Night Cream—which contains the vital hormones.
(Rubinstein advertisement, 1933)
Second, it is possible that the rejuvenating ingredient in the Hormone Night Cream was not oestrogen but rather some other glandular secretion or a hormone precursor such as cholesterol. Winter, for example, recommended cholesterin (cholesterol) be used in ‘nourishing’ and ‘rejuvenating’ creams (Winter, 1932, p. 547).
Rubinstein first mentions oestrogens when she released Gourielli Estrolar Cream in 1942. It contained ‘Glandiol’ a mixture of Estrone and Estradiol. In 1945, she followed this with Estrogenic Hormone Cream and Estrogenic Hormone Oil, replacements for the earlier Hormone Twin Youthifiers.

Above: Helena Rubinstein Estrogenic Hormone Cream and Estrogenic Hormone Oil with measuring spoon.
If Rubinstein did not introduce a true hormone cream until 1942 then the origins of this type of skin-care product becomes contentious.
See also: Helena Rubinstein (1930-1945)
Hormez
The industry magazine, ‘The Drug and Cosmetic Industry’, considered Hormez, to be first oestrogenic hormone cream sold in the American market, not Rubinstein’s Hormone Twin Youthifiers. Produced by Hormez Laboratory – a subsidiary of Louis Phillippe, Inc. – it was released no later than 1932. The magazine notes that it did not generate much interest and suggests that it was not until Hirestra Laboratories launched Endocreme in 1937 that oestrogenic creams began to have a significant impact on the American market (‘Hormone creams’, 1944, p. 289).
See also: Hirestra Laboratories (Endocreme)
Amor skin
Florence Wall puts the first hormone beauty product sold in the United States back at 1927 (Wall, 1961, p. 124), much earlier than Hormez or the Hormone Twin Youthifiers. She did not provide any information about this product but she was probably referring to Amor Skin, developed by Opterapia, a German company with branches in Berlin, Paris, Milan and New York. However, Amor skin was not an oestrogenic cream but rather, appears to have been made with glandular secretions from a species of turtle – although lizards occasionally get mentioned in advertorials. Its primary ingredient was probably turtle oil but the product may have been supplemented with vitamins and other biological extracts.
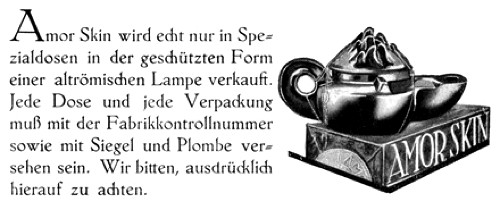
Above: Amor Skin was packaged and sealed in Germany and sold in a reproduction Pompeian lamp for US$16.50 (single strength) or US$25.00 (double strength). By comparison, Helena Rubinstein’s Twin Hormone Youthifiers retailed for US$10.00.
The “upper crust” of American society has known of this marvelous rejuvenating agent for the past several years. Due to the difficulty in securing the proper ingredients the prices was prohibitive for anyone except those who possessed considerable means. Through a fortunate discovery of other sources of supply it is now possible for all of us to take advantage of this remarkable REJUVENATING salve which restores the fresh, youthful, elasticity and smooth unwrinkled tension of the skin. Most of us have noticed either in ourselves or our friends that after, say 25 years of age, our skin does not have quite the dewey freshness of our teens and news of anything that will restore this texture will interest the average woman. Amor Skin is obtained from the areolar tissue of giant lizards and turtles who not only live for centuries, but whose use as a food is considered in some countries to be conducive to the prolongation of life. They also possess truly wonderful regenerative qualities in cases of injury to their shell or the loss of entire limbs or extremities. The tissues of these animals must therefore undoubtedly possess a constructive and regenerative capacity far in excess of the average. The best guarantee of the success of results of Amor Skin is that physicians endorse it for their patients.
(El Paso Evening Post, 1928, p. 28)

Above: 1932 Amor Skin. The likely supplier of the biological material was Dr. Kurt Richter GmbH, a Berlin company established in 1926 that specialised in the production of biologicals (Personal communication, 2017).
Oestrogens
The reason why oestrogens were added skin-care cosmetics is also open to question. Peck and Klarmann make the following suggestion:
Women filling ampoules with oestrogens for intramuscular injections who had frequent contact with the oil-suspended hormones noted that a change in the appearance of the skin on the dorsum of their hands gradually occurred after months of such contact. It seemed that the signs of ageing, such as wrinkling, tended to disappear and the backs of their hands, especially in older women assumed a more youthful appearance. After extensive clinical experimentation (with the hormone-free oil as a control) it was concluded by the observers that the antiwrinkling effects on the skin must have been due primarily to the hormones.
(Peck & Klarmann, 1954, p. 159)
However, Peck and Klarmann place this event at about 1936 which is much later than the introduction of Hormez. In addition, ‘The Chemist and Druggist’ track interest in the use of sex hormones for skin rejuvenation back to 1928.
The ‘Dermatological Weekly’ reports on the rejuvenation of the skin through the absorption of organic substances (1928, No. 87), and also, in another issue of about the same date, on the effect of hormones in accelerated healing after injuries to the skin. The hormones usually incorporated in a cream for outward application are the ovarian and the corpus luteum.
(‘Hormone skin creams’, 1935, p. 354).
‘The Chemist and Druggist’ also published recipe for a hormone cream originally included in the French journal, ‘La Parfumerie Moderne’.
gm Tri-ethyl stearate 175 Glycerin 50 to 100 Olive or almond oil 50 to 100 Female sexual hormones 2 Mammary extract 3 Sodium chloraseptate 0.5 Perfume, a trace. (Hormone skin creams, 1935, p. 354).
Below, I also include some French references to the use of female sex hormones in beauty treatments that are also earlier than the Peck and Klarmann 1936 date.
Above: 1935 Advertisement for medical hormone therapy (French).

Above: Drawing of a hormone applied as a transparent face mask in a Salon de Beauté (Femina, 1934).
Oestrogen sources
Most hormone cosmetic creams produced up until the 1960s that actually contained oestrogens – rather than skin extracts, dried glands, placenta or other materials – consisted of oestrone, oestradiol and oestriol or, if the oestrogens were taken from the urine of pregnant mares, equilin and equilenin. In general it was found that incorporating the hormones into a water-in-oil emulsion worked best – as this allowed for a “slow uniform absorption of the oestrogenic principle” (deNavarre, 1975, p. 628) – or were mixed into an oil.
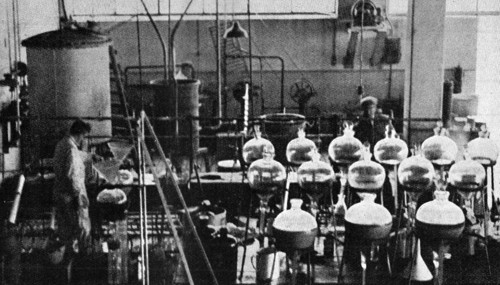
Above: 1936 Manufacturing estrone at the Organon Pharmaceutical Factory in Oss, Holland. Organon began producing female sex hormones in 1925 (Oudshoorn, 1994).

Above: 1948 French laboratory producing hormone extracts.

Above: 1948 Filling ampules with hormone extract in a French laboratory.
In addition to ‘natural oestrogens’ some oestrogenic hormone creams used synthetic compounds with oestrogenic effects such as diethylstilbestrol (DES) first synthesised in 1938. It was cheaper than natural oestrogens, and was also about three times more powerful than the natural hormone.
Hormones were also combined with other biologicals such as vitamins or turtle oil, as for example in the Up-To-Date Cosmetic Company’s Estro-Turtle Hormone Cream which went on sale in the United States in the late 1940s.
See also: Turtle Oil and Vitamin Creams
Early regulation in the United States
In 1938, the Food, Drug and Cosmetic Act (FD&CA) was passed into law in the United States. The American Food and Drug Administration (FDA), who were charged with administering the FD&CA showed a good deal of interest in hormone creams as they crossed the line between drugs and cosmetics. However, as there appeared to be some benefits in using the creams, the FDA was prepared to allow them as long as there was no evidence that they might cause trouble.
In the United States, the “official” position of the Federal Security Agency (Food and Drug Administration) is given in a letter dated 26 October 1949, addressed to deNavarre (Amer. Perfum., April 1950. P. 289) from which it appears that the Food and Drug Administration:
1. whilst they were skeptical of the ability of these creams to accomplish the results promised, were not in a position to disprove their claims;
2. did not look favourably upon the distribution of these creams for lay use (because of definite information concerning the ultimate effect of continued use of these creams), but possessed no conclusive evidence that harm had resulted from the use of creams containing the amounts of estrogens found in these creams then on the market.(Harry, 1955, pp. 99-100)
Whilst the FDA would allow over-the-counter (OTC) hormone creams to remain on the market, they placed limits on the levels of oestrogen allowed in these cosmetics. The FDA was concerned that the hormones in OTC skin creams did not enter the blood stream where they could be transported to other parts of the body with unknown effects.
Scientific research indicated that the absorption of oestrogens by the skin from a cream was less than 40 per cent. So, as long as the concentration of oestrogens in a cream remained low, they did not appear to enter the blood stream and their action remained localised or ‘cosmetic’, i.e., only effected the skin. The FDA appears to have settled on 10,000 International Units (IU) of oestrogens per ounce as an acceptable amount for hormone creams, as long as the amount used was no greater than 2 ounces per month. This limit was supposed to ensure that a user would have a maximum exposure of 670 IU of oestrogens per day. In practice it did not.
It is worth noting that L. Leichner – makers of greasepaints and other cosmetics – agreed with the idea that it was difficult to absorb hormones from skin creams and, at first, supplied their beauty hormones as tablets not cosmetics but changed their mind later.

Above: 1931 Leichner Beauty Hormones. “As it is impossible to administer Hormone preparations to the tissues in the usual form of cosmetics, Leichner Beauty Hormones are supplied in tablets, one tablet should be taken in a little water twice daily, morning and evening.”
See also: Leichner (1910-1945)
The main concern of the FDA was cancer. If oestrogens had been shown to be harmful the FDA would have withdrawn them from the market no matter what the concentration. However, research of the time suggested that any link between OTC hormone creams and cancer would be remote as long as the hormones were not in concentrations high enough to enter the blood stream and circulate to other parts of the body, i.e., they were below the 10,000 IU per ounce limit.
1. It is the consensus of opinion among experience observers that cosmetic hormone creams with a maximum potency of 10,000 IU per ounce (31 g.) of vehicle, if used in the manner by the informed manufacturer, are free from systemic effects. It is also established that to date there has been no evidence of precancerous or cancerous changes in the skin resulting from the use of these hormone cosmetics over a period of several years.
2. There is definite support for the anti-wrinkling effect produced by the use of hormone cosmetics based on (a) the thickening of the epidermis, (b) plumping of the collagen fibers.
3. The anti-wrinkling action of hormone creams is more noticeable in older age-groups.(Peck & Klarmann, 1949, p. 165)
The amount of oestrogens present in American OTC hormone creams differed widely but most fell below 10,000 IU per ounce limit that the FDA used as a safe measure. Some examples are given below:
• Nu-Youth Hormone Day or Night Cream (1,500 IU oestrogens)
• Second Youth Estrogenic Hormone Cream (7,500 IU oestrogens)
• Bette Knowlton Special Formula Cream (50,000 IU oestrogens)
• Revlon White Sable Hormone Liquid Cleansing Creme (6,000 IU oestrogens)
• Allure Fountain of Youth Wrinkleproof Cream (7,500 IU oestrogens)
• Dorothy Gray Cellogen Cream (10,000 IU oestrogens)
When products exceeded the 10,000 IU per ounce the FDA usually confiscated them. In 1956, the FDA seized 223 one-ounce bottles of Hormonex Beauty Serum on the grounds that it could cause injury to health, as the amount of hormone applied was well over the accepted limit. The serum contained 150,000 IU of oestrogens per ounce with directions indicating that the dosage would be 1,500 IU per day. Hormonex subsequently dropped the amount of oestrogens in their beauty serum to 33,000 IU per ounce. This was still higher than 10,000 IU per ounce but as they stipulated a recommended daily dosage of 300 IU per day – which was well below the agreed threshold value for a systemic effect – they overcame the objections of the FDA and remained on the market.
In 1952, the FDA also seized and destroyed 8 jars of Bette Knowlton Special Formula Cream on the grounds that it was dangerous to health. Samples taken indicated it contained 54,000 IU (5.4 mg) of oestrogen per ounce, again well over the 10,000 limit. The directions for use meant that the daily application would be 1,800 IU of oestrogen, leading to the FDA believing that it was possibile that the hormone would enter the blood stream.
The FDA also confiscated products under the Misbranding Section 502(a) of the FD&CA. Here the charge was that claims made for the product could not be supported. This could be because the claims were overly exaggerated or because the hormone levels were so low that the FDA could not see how they could support the claims made for the product. Low-dosage and high-dosage hormone creams were thus both subject to Federal confiscation.
Although the terms of the FD&CA were less than perfect, the United States had the strongest cosmetic regulations at the time. The United Kingdom for example had nothing similar and it was fortunate that most of the hormone creams available there were American in origin.
In America the quantity of œstrogen included in hormone preparations is strictly controlled, by the Federal Food and Drug Administration, at 10.000 IU (International Units) per oz.—this ‘safety figure’ having been arrived at after extensive research. The lowest quantity considered effective is 7,500 IU per oz. Although no control exists in Great Britain, few, if any, beauty preparations are made with more than 10,000 IU per oz. Many of the best known makes of hormone cream are, of course, American in origin, and are formulated accordingly. Many users claim handsomely rewarding results, including a more moist and smoother skin with fresher colour due to improved capillary flow. Other users report no noticeable difference in their complexions.
(Allen, 1961, p. 244)
Progesterone and pregnenolone
By the late 1950s the oestrogenic hormone skin-care market was saturated. For example, Dorothy Gray had released Cellogen, Remoldine and Satura, Elizabeth Arden had Joie de Vivre, and even Max Factor had weighed in with Cup of Youth (1957) sold in a milk-white goblet. In order to differentiate themselves in this crowded market some manufacturers began to add other biological ingredients.
In 1957, Rubinstein added progesterone to her Estrogenic Hormone Cream and then, in 1959, launched her Ultra Feminine Face Cream containing 5 mg of progesterone as well as 10,000 IU of oestrogenic hormones. In 1961, Revlon followed with Eterna 27 containing 0.5 per cent pregnenolone acetate – an ester of the sex hormone precursor pregnenolone – trademarked as Progenitin. Both products were protected by patents.
Both products stressed their scientific development and youthful benefits but used different advertising approaches. The tone of Rubinstein’s advertising copy was drug-like whereas Revlon’s was more cosmetic.
Rubinstein used provocative headlines in her advertising, such as “New Wonder Drug Cosmetic Retores Young Look to Skin” (Rubinstein advertisement, 1959) and made a number of physiological claims for Ultra Feminine including: that “specific skin cells can be reactivated” and that “this wonder drug preparation … works within the skin to replenish hormones dwindled by time”, all claims likely to create problems with the FDA.
Revlon on the other hand only made cosmetic claims for their skin-cream. Advertising copy for Eterna 27 with Progenitin stated that the product had “no hormone activity … no hormone effects” and only claimed that the skin would “look more youthful and definitely smoother”. These were visual/cosmetic not physiological claims and the product did not come under any regulatory pressure.
The decision by Revlon to market Eterna 27 using language deemed appropriate for a cosmetic rather than a drug can perhaps serve as a marker for the beginning of the end for OTC hormone creams. From now on the regulatory landscape, both in the United States and elsewhere, would progressively turn against them. Hostility to the use of hormones in OTC products was, in part, driven by the improved scientific understanding that followed the introduction of the contraceptive pill in 1960 and the increased use of Hormone Replacement Therapy (HRT).
The complete ban on the use of hormones or hormone precursors in OTC products by the European Commission began with the introduction of the Council Directive 76/768/EEC in 1976. Although this excluded some hormones the objective of the directive was clear.
The FDA published proposals for regulation of OTC hormone products in 1962 but its final rules were not published until 1993. However, the costs associated with FDA compliance and labeling requirements, and lack of access to European and other markets would have been strong incentives to remove hormones from skin-care products well before this. Cosmetic companies moved on to less-regulated compounds like phytosterols found in extracts from plants such as avocado oil, and other so-called bio-identical oestrogens. Of all the hormone creams and oils produced by the majors mentioned here, only Revlon’s Eterna 27 with pregnenolone acetate remained on the market at the end of the century.

Above: Revlon Eterna 27 with Progenitin ingredient list.
I should add that the European Commission banned the use of pregnenolone acetate in skin creams in 1991.
See also: Placental Creams and Serums
First Posted: 17th February 2011
Last Update: 16th October 2020
Sources
Allen, E. (Ed.). (1961). The book of beauty. London: George Newnes.
deNavarre, M. G. (1941). The chemistry and manufacture of cosmetics. Boston: D Van Nostrand Company.
deNavarre, M. G. (1975). The chemistry and manufacture of cosmetics (2nd. ed.). Orlando: Continental Press.
Ebling. F. J. (1974). Sex hormones and skin. Journal of the Society of Cosmetic Chemists of Great Britain. 25. 381-395.
European Commission. (n.d.) Council Directive of 27 July 1976 on the approximation of the laws of the Member States relating to cosmetic products (76/768/EEC). Retrieved September 16, 2018, from: https://publications.europa.eu/en/publication-detail/-/publication/5236f8c3-3f55-4684-bed3-f17774905383/language-en
Food & Drug Administration. (n.d.). Rulemaking History for OTC Hormone Drug Products. Retrieved September 16, 2018, from: https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/Over-the-CounterOTCDrugs/StatusofOTCRulemakings/ucm071333.htm
Harry, R. G. (1940). Modern cosmeticology: The principles and practices of modern cosmetics. Brooklyn, NY: Chemical Publishing Company.
Harry, R. G. (1955). Modern cosmeticology (4th ed.). London: Leonard Hill.
Helena Rubinstein. (1932). The hormone twins [Booklet]. USA: Author.
Hormone creams. (1944). The Drug and Cosmetic Industry, May, 54(3), 289-290.
Hormone skin creams. (1935). The Chemist and Druggist. September 21, 354.
Les col’hormones. (1934). Femina. January, 28.
Oudshoorn, N. (1994). Beyond the natural body: An archeology of sex hormones. London: Routledge.
Peck, S. M., & Klarmann, E. G. (1954). Hormone cosmetics. The Practitioner. 173. 159-165.
Sagarin, E. (Ed.). (1957). Cosmetics: Science and technology. New York: Interscience Publishers, Inc.
Schlossman, M. L. (Ed.). (2000). The chemistry and manufacture of cosmetics.. Carol Stream, Il: Allured Publishing corporation.
Strauss, J. S. (1963). Hormones in cosmetics. Journal of the American Medical Association. 186(8). 99-102.
Wells, F. V., & Lubowe, I. I. (1964). Cosmetics and the skin. New York: Reinhold Publishing Corporation.
Wall, F. E. (1961). The principles and practice of beauty culture (4th ed.). New York: Keystone Publications.
Winter, F. (1932). Handbuch der gesamten parfumerie und kosmetik (2nd ed.). Vienna: Springer-Verlag.
Woodhead, L. (2003). War paint: Miss Elizabeth Arden and Madame Helena Rubinstein. Their lives, their times, their rivalry. London: Virago.

Hormez containing oestrogenic hormones.
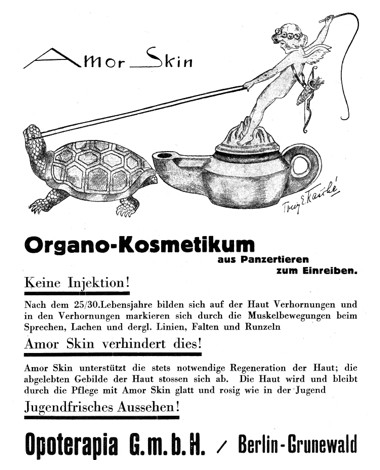
1928 Amor Skin. The name of the product literally means cupid skin. The turtle is the source of the extract. No injections required!

1929 Amor Skin as distributed in the United States by the Amorskin Corporation of New York from 1927.

1932 Helena Rubinstein – Hormone Twin Youthifiers.

1932 Dr. Ballowitz & Co. W-5 Dragees. These anti-wrinkle pills containing hormones were formulated by Dr. Joseph Kapp of Berlin and Wiesbaden. Helena Rubinstein frequently referred to him in the post-war advertising for her hormone cosmetics but situates him in Vienna.

1934 Elizabeth Arden Joie de Vivre (Gland Cream).

1937 Hormone face mask from Fernand Aubry.

1938 Académie Scientific de Beauté Vivormones.

1939 Hormone Laboratories.

1944 Helena Rubinstein Gourielli Estrolar Cream.

1946 Harper Method Beauty HarperGENE Cream and HarperGENE Oil containing oestrogenic hormones.

Urine collector attached to a pregnant mare. A number of animal rights organisations have been agitating to have the practice banned.

1947 Nu-Youth Hormone Creme with 7,500 IU per ounce of natural oestrogens.

1947 Bonnie Bell Plus 30.

1948 Endoceme Hormone Cream.

1948 Salon hormone treatment (France). After deep cleansing, a lotion is applied which lightly exfoliates the face and produces a slight vaso-dilation to help improve the penetration of the hormonal product. The hormonal liquid is brushed over the face twice before being covered with a plastic mask to help penetration and reduce the possibility of it being oxidised by the air.

1949 Elizabeth Arden Joie de Vivre with oestrogenic substances

1950 Helena Rubinstein Estrogenic Hormone Cream and Oil.

1954 Dorothy Gray Remoldine.

1957 Hormonex, first introduced in 1954.

1957 Gala Hormone Series consisting of a Hormone Day Cream, Hormone Night Cream and Hormone Lotion.

1958 Organon Laboratories Endocil.

1959 Max Factor Cup of Youth.

1962 Pohli Hormon-creme.

1962 Revlon Eterna 27 with Progenitin.

1963 Hormocenta.
