Acid Creams
In the 1890s science discovered that the skin had an acidic surface. Then, in 1928, Schade and Marchionini suggested that the function of the low pH film was to protect the skin from microbes and coined the term ‘acid mantle’ to describe it. Even though they did not explain how the acid mantle achieved its protective effect, their idea was largely accepted and repeated throughout the scientific literature of the day.
Alkaline soaps
Many soaps and detergents of the time were quite alkaline in nature, and their harmful effects on the skin were well known by consumers and dermatologists alike; so the concept of the acid mantle went some way to explain why this was the case. You might think that cosmetic companies would jump on this fact and use it to persuade consumers to exchange soap for a skin cleanser but this does not appear to have been the case. One possible reason for this is that many cosmetic companies were also in the soap business, with some of them also using soap in their cleansing routines. In addition, most cosmetic houses were also fighting a cleansing war on two fronts. As well as suggesting that consumers use cleansers rather than soap, they were also trying to wean them off cleansing with cold cream. To do this they needed to convince their customers that a specialist cleanser provided a better solution.
The Helena Rubinstein scientific washing preparations accomplish what could never be done by creams or soap alone! They not only cleanse deeply—they impart an animated, transparent look to the skin.
(Helena Rubinstein brochure, 1950s)
Rather than take a negative approach, beauty firms concentrated on the more positive ‘soothing’, ‘feeding’, ‘beautifying’, and ‘nourishing’ aspects of their products, thus avoiding unpleasant subjects like acids and microbes.
Alkalinity and acidity only get mentioned in advertisements for cosmetic products where the pH could be considered to be a potential problem, namely in soaps and shampoos; however, this marketing trend did not begin to appear until later in the twentieth century. It was not that cosmetic companies were unaware of the presence of the acid mantle or the harmful effects of alkaline materials on the skin, it’s just that most did not consider it to be a good selling point. Even so, many thought it prudent to keep the pH of their products as close to that of the skin or as neutral as possible.
In regard to negative advertising, one soap company was not so squeamish. Despite protests from cosmetic houses, Lever Brothers took to describing the dangers of acquiring ‘Cosmetic Skin’ in their advertisements for Lux Toilet Soap; the cure for which was Lux Soap and water; apparently, “[r]omance comes to the girl who guards against Cosmetic Skin”. Lever Brothers ran this marketing line in their Lux advertising for a few years in the 1930s using a range of stage and screen stars as support.

Above: 1939 Lux soap advertisement. “It is when cosmetics choke the pores that they cause Cosmetic Skin. Enlarged pores—tiny blemishes—a dull, lifeless look—these are warning signs that you are not removing cosmetics properly.”
See also: Cosmetic Skin
Marketing acidity
Although it was ignored in advertising by most cosmetic companies, some did use the concept of acidity as a marketing tool. Two different approaches were tried, neither of which was particularly successful.
The first approach was to suggest that an acid skin needed treatment. In the 1920s, Princess Pat expressed concern about an acidic ‘pore film’ created by a build up of fatty acid.
Acid skin must be overcome
When perspiration and the oil from the sebaceous glands intermingle and decompose, there is formed upon the skin a fatty acid which is highly favourable to the formation of blackheads, whiteheads pimples and other skin blemishes. To the extent that the glands of the skin are enfeebled and unhealthy, this fatty acid becomes Increasingly a source of trouble. It lingers on the skin mouths and together with accumulations of dust, dirt and germs from the air exerts a most disastrous effect.(Princess Pat, 1923, pp. 10-11)
In the 1930s, Philips tried something similar by advocating the use of creams containing Milk of Magnesia (emulsified magnesium hydroxide) to neutralise any skin acid (deNavarre, 1941, p. 133). The company was ordered to stop using the term acid skin by the American Federal Trade commission (FTC) in 1941, on the grounds that the term did not describe any known skin disease or pathological condition.

Above: 1938 Lili Christine Beauty Products.
Lili Christine Cosmetics went one step further and added a pH indicator to their Acid Removal Cream. The pH of the cream was neutral (green) but it would turn ivory then pink when it came in contact with the acid mantle of the skin.
The Acid Removal Cream—the name gives its purpose—reacts like a litmus paper. In the jar it’s green, but if your skin is slightly acid when you apply it, it turns to an ivory shade; and if there is a lot of acid, it turns pink!
(Lili Christine advertisement, 1938)
Rather than fight the acid mantle, a second approach was to support it by manufacturing and marketing cosmetics with an acidic pH. This was the approach Frances Denney took when marketing its Formula A-B-C introduced in 1951.
When the acid of the skin is either “plus” or “minus” – beware! Yor beauty can be marred and your lovely complexion may vanish. Then you must find the secret of normalizing.
The skin itself is acidic. Many things, however, can cause the skin to vary from its normal acidity – diet, illness, weather and other causes. The skin may become “plus” or “minus”.
A skin with more than normal acidity is a “plus” skin . . . and this skin is not beautiful. An indication of “plus” acidity may be blotches, redness, irritation, moist surface blemishes. A “minus” skin is skin with less than normal acidity. If you are troubled with “minus” acidity your skin may be flaky, irritated, have dry surface blemishes.
To normalize acidity, whether “plus” or “minus” there is a new tested cosmetic formula. It is called formula A-B-C and is presented by Frances Denney.(Frances Denney advertisement, 1952)
In 1957, Charles of the Ritz used the idea of the acid/alkaline balance to promote his new Treatment Lipstick. As well as moisturising, the lipstick was also said to avoid colour changes due to ‘alkaline deterioration’.
[A] cosmetic “first”—first time lipstick has made a point of using an acid rather than an alkaline base. By Charles of the Ritz and called Treatment Lipstick, it’s a case of dry-skin prevention rather than cure, uses a special moisturizing agent plus (and it’s a big plus)—the Acid-pH factor already at work in creams and hand lotions. Added advantage of Acid-pH here: it keeps the colour of the lipstick in the case true on the lips (i.e., no alkaline deterioration).
(Charles of the Ritz advertorial, 1957).
See also: Charles of the Ritz (post 1945)
Formulation
When developing an acid cream, chemists had to keep in mind that mild acids decompose soap so alternative emulsifying agents had to be used. Two suggested formulas are given below:
Acid Cream 2 % Sodium cetyl sulfate 4.0 Cetyl alcohol 8.0 Ozokerite, bleached 2.5 Mineral oil 12.0 Diethylene glycol 5.0 Lemon perfume compound (for creams) 0.5 Glycerophosphoric acid 2.5 Distilled water 65.5 (Thomssen, 1947, p. 193)
Formula 27 % Wool wax 3.0 Lanolin, anhydrous 8.0 Stearyl alcohol 2.0 Petrolatum 37.0 Glycerol 4.0 Lactic acid 1.5 Water 44.5 Procedure: Melt the wool wax, lanolin, stearyl alcohol, and petrolatum and bring to a temperature of 55°C. Add glycerol and lactic acid to the water and heat to 57°C. Add the water solution to the fats with good agitation. When all the water has been added, continue stirring until the temperature has dropped to 45 to 50°C., and then add the perfume. Pass the cream through a colloid mill (Eppenbach type).
(Sagarin, 1957, p. 92)
There were also marketing difficulties in selling these creams. Consumers generally did not like the idea of putting acid on their skin. This is still true today with many acidic products, like shampoos, still being referred to as non-alkaline rather than acidic. For this reason acid creams were sometimes marketed as ‘skin normalisers’ or as ‘lemon creams’. If the term lemon cream was used the formula had to contain citric acid or be perfumed with oil of lemon to avoid problems with regulatory authorities.
Acid creams never really caught on with the public and are restricted today to quasi-medical creams for individuals with specific skin conditions. Meanwhile, many manufacturers of soaps and shampoos had learnt their lesson and began advertising their products as ‘alkali-free’ or ‘non-alkaline’.
Alpha-hydroxy acids
Acidic cosmetics ‘time in the sun’ had to wait until the end of the twentieth century, when alpha-hydroxy acids (AHAs) began to be added to cosmetic products. Marketed as ‘fruit acids’ these were generally milder versions of a type of chemical peel employed by dermatologists and plastic surgeons to remove the signs of skin roughness, ageing and pigmentation. Like many other beauty products, they moved from the medical profession to the salon and then to general distribution over the counter. In their heyday they penetrated all aspects of the skin-care market including cleansers, moisturisers and masks.
Typically AHAs, such as glycolic, malic, citric and lactic acids, made up less than 10% of the formulation. This was not always the case and products with much higher percentages were made up for use in salons with some establishments making up solutions on the spot. The pH of the products also varied but it was generally agreed that commercial products should not get down below a pH of 3.0 (Winter, 2005, p. 61). I doubt that all salons stuck to this limit when making up their own solutions.
Being acidic, these products – and the BHAs (beta-hydroxy-acids) that followed them – could be dangerous if a formulation with a very low pH was left on the skin too long. The discomfort that could occur when undergoing some fruit-acid treatments was a measure of how far consumers would go to get younger-looking skin. Fortunately, as with alkaline soaps, as long as the exposure was short and the skin was not damaged, natural body processes soon restored the acid mantle returning the skin’s pH to normal levels.
First Posted: 16th September 2009
Last Update: 3rd March 2023
Sources
deNavarre, M. G. (1941). The chemistry and manufacture of cosmetics. Boston: D. Van Nostrand Company.
Poucher, W. A. (1932). Perfumes, cosmetics and soaps, Vols. 1-2 (4th ed.). London: Chapman and Hall.
Princess Pat. (1923). The promise of a princess [Booklet]. Chicago, IL: Author.
Sagarin, E. (Ed.). (1957). Cosmetics: Science and technology. New York: Interscience Publishers, Inc.
Thomssen, B. S. (1947). Modern cosmetics (3rd ed.). New York: Drug & Cosmetic Industry.
Wilkinson J. B., & Moore, R. J. (Eds.). (1982). Harry’s cosmetology (7th ed.). New York: Chemical Publishing.
Winter R. (2005). A Consumers dictionary of cosmetic ingredients (6th. ed.). New York: Three Rivers Press.

Heinrich Karl Wilhelm Schade [1876-1935].

Alfred Marchionini [1899-1965].
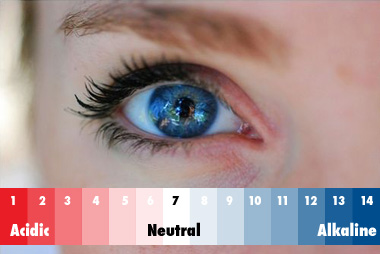
The normal pH of the surface of the skin is 4-6 which means it is acidic.

1927 Princess Pat Skin Cleanser to counteract acidic ‘pore film’.

1932 Frances Hemming of Cyclax promised to remove acid waste collecting beneath the surface of the skin that was the cause of the skin developing a yellowish tinge. It is unlikely that she was referring to the acid mantle but rather had a skin equivalent of urine in mind. Special Lotion was supposed to draw this out along with other ‘skin poisons’.

1936 Helena Rubinstein Poudre Évolution and Créme Évolution. Both cosmetics were advertised as supporting the natural acidity of the skin and the beauty and health of the epidermis.

1938 Monsavon pH6. Rather than being neutral or alkaline this soap was slightly acidic.

1939 Phillip’s Magnesia Texture Cream and Cleansing Cream. The company was issued with a cease and desist order by the FTC in 1941.

The German company Beiersdorf, makers of Nivea and Elastoplast, first marketed Ph5 Eucerin cream in 1950. The packaging indicates the cream was seen as a pharmaceutical product rather than a cosmetic.

Above: 1954 Frances Denney fFormula A-B-C.

1954 Placid Cleansing Cream and Skin Lotion.

1962 Bonnie Bell Ten·O·Six – has the same acid/alkaline ratio as normal skin.
